σ2-Adaptin Facilitates Basal Synaptic Transmission and Is Required for Regenerating Endo-Exo Cycling Pool Under High-Frequency Nerve Stimulation in Drosophila
- PMID: 26920756
- PMCID: PMC4858786
- DOI: 10.1534/genetics.115.183863
σ2-Adaptin Facilitates Basal Synaptic Transmission and Is Required for Regenerating Endo-Exo Cycling Pool Under High-Frequency Nerve Stimulation in Drosophila
Abstract
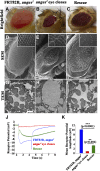
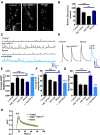
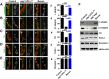
The functional requirement of adapter protein 2 (AP2) complex in synaptic membrane retrieval by clathrin-mediated endocytosis is not fully understood. Here we isolated and functionally characterized a mutation that dramatically altered synaptic development. Based on the aberrant neuromuscular junction (NMJ) synapse, we named this mutation angur (a Hindi word meaning "grapes"). Loss-of-function alleles of angur show more than twofold overgrowth in bouton numbers and a dramatic decrease in bouton size. We mapped the angur mutation to σ2-adaptin, the smallest subunit of the AP2 complex. Reducing the neuronal level of any of the subunits of the AP2 complex or disrupting AP2 complex assembly in neurons phenocopied the σ2-adaptin mutation. Genetic perturbation of σ2-adaptin in neurons leads to a reversible temperature-sensitive paralysis at 38°. Electrophysiological analysis of the mutants revealed reduced evoked junction potentials and quantal content. Interestingly, high-frequency nerve stimulation caused prolonged synaptic fatigue at the NMJs. The synaptic levels of subunits of the AP2 complex and clathrin, but not other endocytic proteins, were reduced in the mutants. Moreover, bone morphogenetic protein (BMP)/transforming growth factor β (TGFβ) signaling was altered in these mutants and was restored by normalizing σ2-adaptin in neurons. Thus, our data suggest that (1) while σ2-adaptin facilitates synaptic vesicle (SV) recycling for basal synaptic transmission, its activity is also required for regenerating SVs during high-frequency nerve stimulation, and (2) σ2-adaptin regulates NMJ morphology by attenuating TGFβ signaling.
Keywords: Drosophila; angur; pMAD; physiology; synapse.
Copyright © 2016 by the Genetics Society of America.
Figures





References
-
- Aberle H., Haghighi A. P., Fetter R. D., McCabe B. D., Magalhaes T. R., et al. , 2002. wishful thinking encodes a BMP type II receptor that regulates synaptic growth in Drosophila. Neuron 33: 545–558. - PubMed
-
- Brand A. H., Perrimon N., 1993. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118: 401–415. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

