Vaginal progesterone prophylaxis for preterm birth (the OPPTIMUM study): a multicentre, randomised, double-blind trial
- PMID: 26921136
- PMCID: PMC5406617
- DOI: 10.1016/S0140-6736(16)00350-0
Vaginal progesterone prophylaxis for preterm birth (the OPPTIMUM study): a multicentre, randomised, double-blind trial
Erratum in
-
Department of Error.Lancet. 2019 Jan 19;393(10168):228. doi: 10.1016/S0140-6736(18)32625-4. Lancet. 2019. PMID: 30663595 Free PMC article. No abstract available.
-
Department of Error.Lancet. 2019 Apr 20;393(10181):1596. doi: 10.1016/S0140-6736(19)30860-8. Lancet. 2019. PMID: 31007201 Free PMC article. No abstract available.
Abstract
Background: Progesterone administration has been shown to reduce the risk of preterm birth and neonatal morbidity in women at high risk, but there is uncertainty about longer term effects on the child.
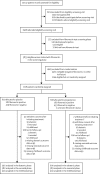
Methods: We did a double-blind, randomised, placebo-controlled trial of vaginal progesterone, 200 mg daily taken from 22-24 to 34 weeks of gestation, on pregnancy and infant outcomes in women at risk of preterm birth (because of previous spontaneous birth at ≤34 weeks and 0 days of gestation, or a cervical length ≤25 mm, or because of a positive fetal fibronectin test combined with other clinical risk factors for preterm birth [any one of a history in a previous pregnancy of preterm birth, second trimester loss, preterm premature fetal membrane rupture, or a history of a cervical procedure to treat abnormal smears]). The objective of the study was to determine whether vaginal progesterone prophylaxis given to reduce the risk of preterm birth affects neonatal and childhood outcomes. We defined three primary outcomes: fetal death or birth before 34 weeks and 0 days gestation (obstetric), a composite of death, brain injury, or bronchopulmonary dysplasia (neonatal), and a standardised cognitive score at 2 years of age (childhood), imputing values for deaths. Randomisation was done through a web portal, with participants, investigators, and others involved in giving the intervention, assessing outcomes, or analysing data masked to treatment allocation until the end of the study. Analysis was by intention to treat. This trial is registered at ISRCTN.com, number ISRCTN14568373.
Findings: Between Feb 2, 2009, and April 12, 2013, we randomly assigned 1228 women to the placebo group (n=610) and the progesterone group (n=618). In the placebo group, data from 597, 587, and 439 women or babies were available for analysis of obstetric, neonatal, and childhood outcomes, respectively; in the progesterone group the corresponding numbers were 600, 589, and 430. After correction for multiple outcomes, progesterone had no significant effect on the primary obstetric outcome (odds ratio adjusted for multiple comparisons [OR] 0·86, 95% CI 0·61-1·22) or neonatal outcome (OR 0·62, 0·38-1·03), nor on the childhood outcome (cognitive score, progesterone group vs placebo group, 97·3 [SD 17·9] vs 97·7 [17·5]; difference in means -0·48, 95% CI -2·77 to 1·81). Maternal or child serious adverse events were reported in 70 (11%) of 610 patients in the placebo group and 59 (10%) of 616 patients in the progesterone group (p=0·27).
Interpretation: Vaginal progesterone was not associated with reduced risk of preterm birth or composite neonatal adverse outcomes, and had no long-term benefit or harm on outcomes in children at 2 years of age.
Funding: Efficacy and Mechanism Evaluation (EME) Programme, a Medical Research Council (MRC) and National Institute for Health Research (NIHR) partnership. The EME Programme is funded by the MRC and NIHR, with contributions from the Chief Scientist Office in Scotland and National Institute for Social Care and Research in Wales.
Copyright © 2016 Norman et al. Open Access article distributed under the terms of CC BY. Published by Elsevier Ltd.. All rights reserved.
Figures
Comment in
-
Progestogens and preterm birth--not the hoped for panacea?Lancet. 2016 May 21;387(10033):2066-2068. doi: 10.1016/S0140-6736(16)00543-2. Epub 2016 Feb 24. Lancet. 2016. PMID: 26921135 No abstract available.
-
Progesterone has no place in the prevention of preterm delivery: FOR: It is time to study something else.BJOG. 2016 Aug;123(9):1510. doi: 10.1111/1471-0528.14159. BJOG. 2016. PMID: 27440592 No abstract available.
-
Progesterone has no place in the prevention of preterm delivery: AGAINST: A call for a measured response to the OPPTIMUM trial.BJOG. 2016 Aug;123(9):1511. doi: 10.1111/1471-0528.14161. BJOG. 2016. PMID: 27440593 No abstract available.
-
Vaginal progesterone prophylaxis for preterm birth.Lancet. 2016 Sep 17;388(10050):1159-60. doi: 10.1016/S0140-6736(16)31614-2. Epub 2016 Sep 16. Lancet. 2016. PMID: 27650091 No abstract available.
-
Vaginal progesterone prophylaxis for preterm birth.Lancet. 2016 Sep 17;388(10050):1159. doi: 10.1016/S0140-6736(16)31612-9. Epub 2016 Sep 16. Lancet. 2016. PMID: 27650092 No abstract available.
-
Vaginal progesterone prophylaxis for preterm birth - Author's reply.Lancet. 2016 Sep 17;388(10050):1160. doi: 10.1016/S0140-6736(16)31016-9. Epub 2016 Sep 16. Lancet. 2016. PMID: 27650093 No abstract available.
References
-
- Romero R, Nicolaides K, Conde-Agudelo A. Vaginal progesterone in women with an asymptomatic sonographic short cervix in the midtrimester decreases preterm delivery and neonatal morbidity: a systematic review and metaanalysis of individual patient data. Am J Obstet Gynecol. 2012;206:e1–19. - PMC - PubMed
-
- Soule S. Meeting of the Advisory Committee for Reproductive Health Drugs. Jan 20, 2012. http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMateria... (accessed Feb 4, 2016).
-
- Progesterone and preterm birth prevention: translating clinical trials data into clinical practice. Am J Obstet Gynecol. 2012;206:376–386. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

