Mcl-1 confers protection of Her2-positive breast cancer cells to hypoxia: therapeutic implications
- PMID: 26921175
- PMCID: PMC4769490
- DOI: 10.1186/s13058-016-0686-4
Mcl-1 confers protection of Her2-positive breast cancer cells to hypoxia: therapeutic implications
Erratum in
-
Correction: Mcl-1 confers protection of Her2-positive breast cancer cells to hypoxia: therapeutic implications.Breast Cancer Res. 2024 Apr 2;26(1):58. doi: 10.1186/s13058-024-01811-y. Breast Cancer Res. 2024. PMID: 38566222 Free PMC article. No abstract available.
Abstract
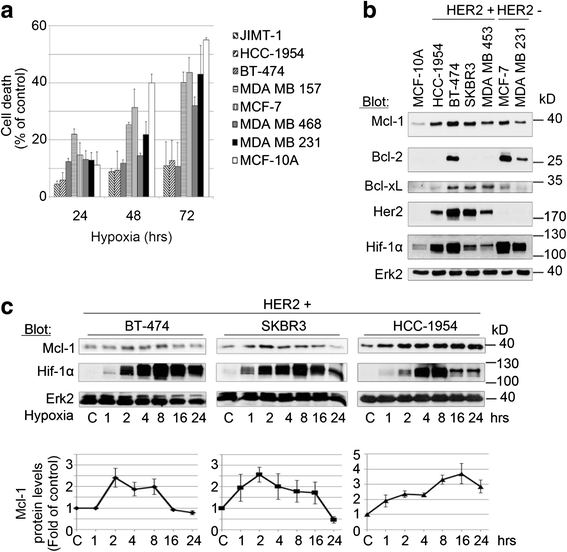
Background: Molecular mechanisms leading to the adaptation of breast cancer (BC) cells to hypoxia are largely unknown. The anti-apoptotic Bcl-2 family member myeloid cell leukemia-1 (Mcl-1) is frequently amplified in BC; and elevated Mcl-1 levels have been correlated with poor prognosis. Here we investigated the pathophysiologic role of Mcl-1 in Her2-positive BC cells under hypoxic conditions.
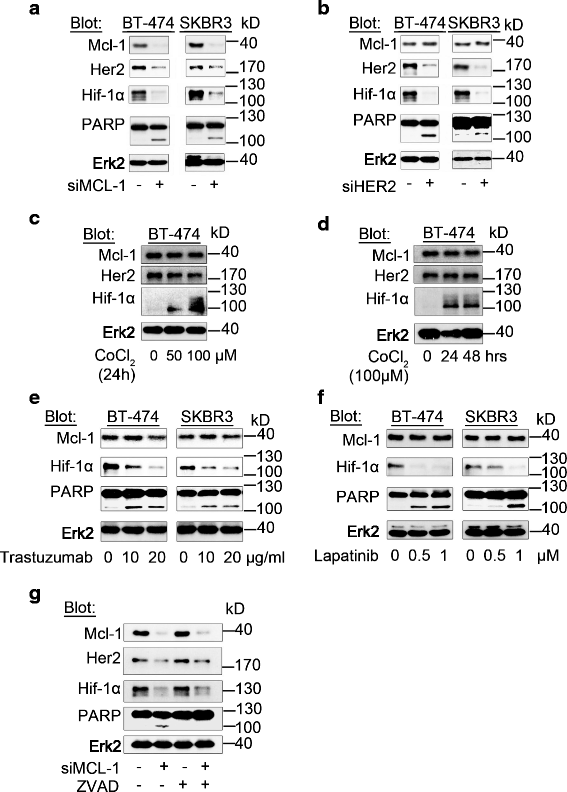
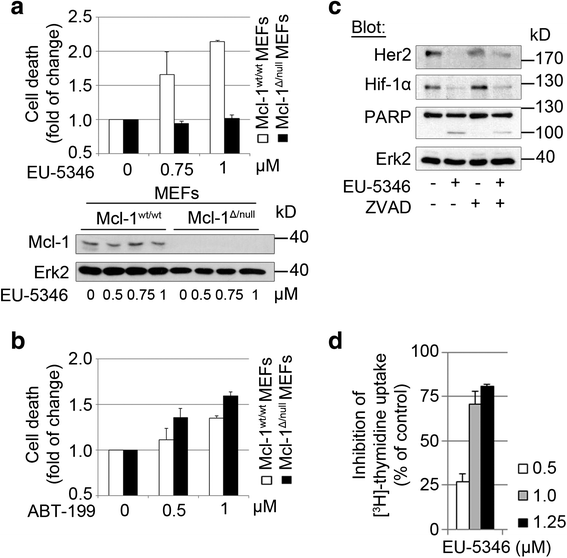
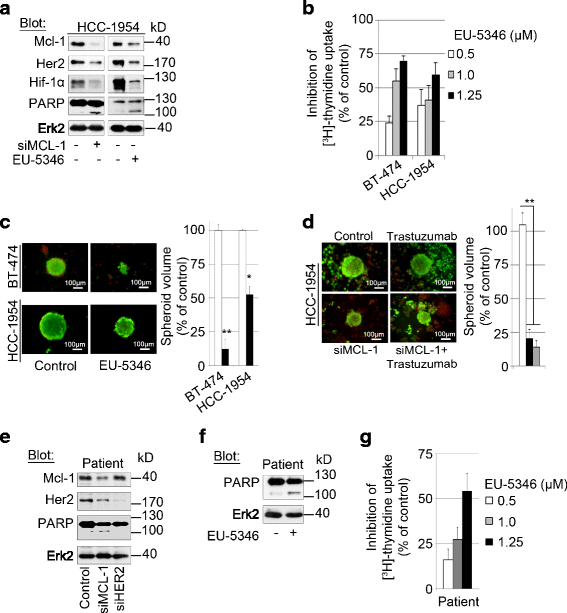
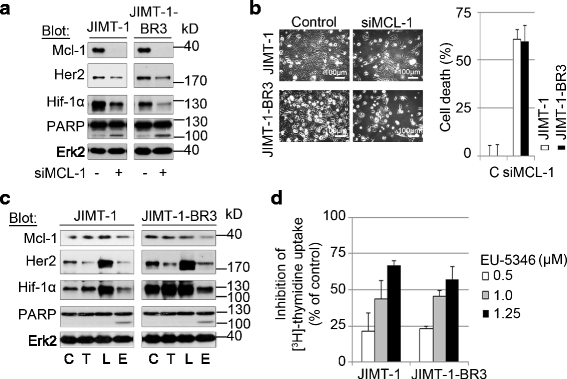
Methods: RNA interference and a novel small molecule inhibitor, EU-5346, were used to examine the role of Mcl-1 in Her2-positive BC cell lines and primary BC cells (sensitive or intrinsically resistant to Her2 inhibitors) under hypoxic conditions (using a hypoxic incubation chamber). Mechanisms-of-action were investigated by RT-PCR, mitochondrial isolation, as well as immunoprecipitation/blotting analysis, and microscopy. The specificity against Mcl-1 of the novel small molecule inhibitor EU5346 was verified in Mcl-1(Δ/null) versus Mcl-1(wt/wt) Murine Embryonic Fibroblasts (MEFs). Proliferation, survival, and spheroid formation were assessed in response to Mcl-1 and Her2 inhibition.
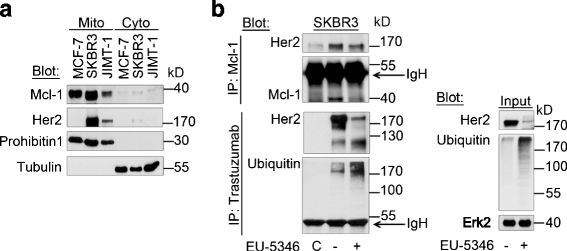
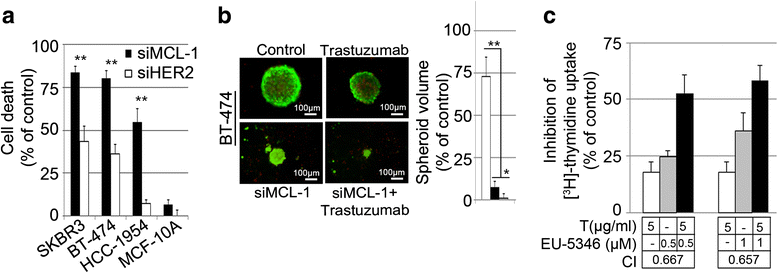
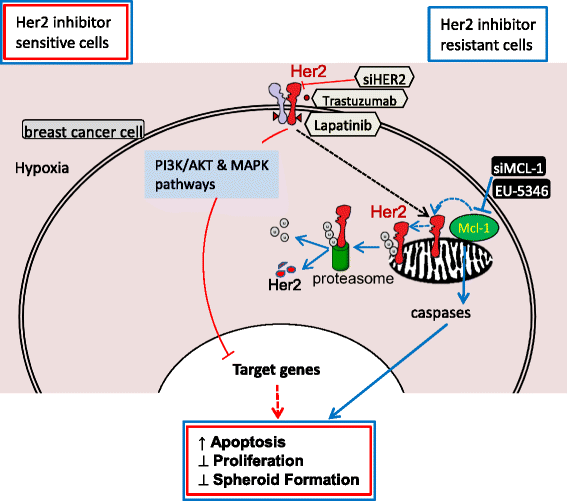
Results: We demonstrate for a strong correlation between high Mcl-1 protein levels and hypoxia, predominantly in Her2-positive BC cells. Surprisingly, genetic depletion of Mcl-1 decreased Her2 and Hif-1α levels followed by inhibition of BC cell survival. In contrast, Mcl-1 protein levels were not downregulated after genetic depletion of Her2 indicating a regulatory role of Mcl-1 upstream of Her2. Indeed, Mcl-1 and Her2 co-localize within the mitochondrial fraction and form a Mcl-1/Her2- protein complex. Similar to genetically targeting Mcl-1 the novel small molecule Mcl-1 inhibitor EU-5346 induced cell death and decreased spheroid formation in Her2-positive BC cells. Of interest, EU-5346 induced ubiquitination of Mcl-1- bound Her2 demonstrating a previously unknown role for Mcl-1 to stabilize Her2 protein levels. Importantly, targeting Mcl-1 was also active in Her2-positive BC cells resistant to Her2 inhibitors, including a brain-primed Her2-positive cell line.
Conclusion: Our data demonstrate a critical role of Mcl-1 in Her2-positive BC cell survival under hypoxic conditions and provide the preclinical framework for the therapeutic use of novel Mcl-1- targeting agents to improve patient outcome in BC.
Figures
Similar articles
-
Rationally derived drug combinations with the novel Mcl-1 inhibitor EU-5346 in breast cancer.Breast Cancer Res Treat. 2019 Feb;173(3):585-596. doi: 10.1007/s10549-018-5022-5. Epub 2018 Oct 29. Breast Cancer Res Treat. 2019. PMID: 30374681
-
c-Myc dependent expression of pro-apoptotic Bim renders HER2-overexpressing breast cancer cells dependent on anti-apoptotic Mcl-1.Mol Cancer. 2011 Sep 7;10:110. doi: 10.1186/1476-4598-10-110. Mol Cancer. 2011. PMID: 21899728 Free PMC article.
-
Targeting transcription of MCL-1 sensitizes HER2-amplified breast cancers to HER2 inhibitors.Cell Death Dis. 2021 Feb 15;12(2):179. doi: 10.1038/s41419-021-03457-6. Cell Death Dis. 2021. PMID: 33589591 Free PMC article.
-
Key Survival Factor, Mcl-1, Correlates with Sensitivity to Combined Bcl-2/Bcl-xL Blockade.Mol Cancer Res. 2017 Mar;15(3):259-268. doi: 10.1158/1541-7786.MCR-16-0280-T. Epub 2016 Dec 30. Mol Cancer Res. 2017. PMID: 28039357 Free PMC article.
-
Hypoxia/HIF1α induces lapatinib resistance in ERBB2-positive breast cancer cells via regulation of DUSP2.Oncotarget. 2015 Feb 10;6(4):1967-80. doi: 10.18632/oncotarget.2806. Oncotarget. 2015. PMID: 25596742 Free PMC article.
Cited by
-
3-Chloroplumbagin Induces Cell Death in Breast Cancer Cells Through MAPK-Mediated Mcl-1 Inhibition.Front Pharmacol. 2019 Jul 26;10:784. doi: 10.3389/fphar.2019.00784. eCollection 2019. Front Pharmacol. 2019. PMID: 31404252 Free PMC article.
-
The role of hypoxia-inducible factor-1 alpha in multidrug-resistant breast cancer.Front Oncol. 2022 Aug 8;12:964934. doi: 10.3389/fonc.2022.964934. eCollection 2022. Front Oncol. 2022. PMID: 36003773 Free PMC article. Review.
-
Myeloid cell leukemia 1 (MCL-1), an unexpected modulator of protein kinase signaling during invasion.Cell Adh Migr. 2018;12(6):513-523. doi: 10.1080/19336918.2017.1393591. Epub 2017 Dec 21. Cell Adh Migr. 2018. PMID: 29166822 Free PMC article. Review.
-
Unraveling the triad of hypoxia, cancer cell stemness, and drug resistance.J Hematol Oncol. 2025 Mar 18;18(1):32. doi: 10.1186/s13045-025-01684-4. J Hematol Oncol. 2025. PMID: 40102937 Free PMC article. Review.
-
Inhibition of Anti-Apoptotic Bcl-2 Proteins in Preclinical and Clinical Studies: Current Overview in Cancer.Cells. 2020 May 21;9(5):1287. doi: 10.3390/cells9051287. Cells. 2020. PMID: 32455818 Free PMC article. Review.
References
-
- Inwald EC, Klinkhammer-Schalke M, Hofstädter F, Zeman F, Koller M, Gerstenhauer M, et al. Ki-67 is a prognostic parameter in breast cancer patients: results of a large population-based cohort of a cancer registry. Breast Cancer Res Treat. 2013;139:539–52. doi: 10.1007/s10549-013-2560-8. - DOI - PMC - PubMed
-
- Ellis MJ, Perou CM. The genomic landscape of breast cancer as a therapeutic roadmap. Cancer Discov. 2013;3:27–34. doi: 10.1158/2159-8290.CD-12-0462. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

