Co-fibrillogenesis of Wild-type and D76N β2-Microglobulin: THE CRUCIAL ROLE OF FIBRILLAR SEEDS
- PMID: 26921323
- PMCID: PMC4850305
- DOI: 10.1074/jbc.M116.720573
Co-fibrillogenesis of Wild-type and D76N β2-Microglobulin: THE CRUCIAL ROLE OF FIBRILLAR SEEDS
Abstract
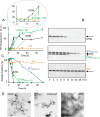
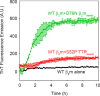
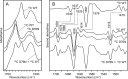
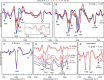
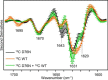
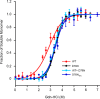
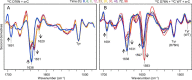
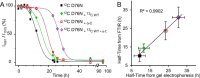
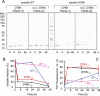
The amyloidogenic variant of β2-microglobulin, D76N, can readily convert into genuine fibrils under physiological conditions and primes in vitro the fibrillogenesis of the wild-type β2-microglobulin. By Fourier transformed infrared spectroscopy, we have demonstrated that the amyloid transformation of wild-type β2-microglobulin can be induced by the variant only after its complete fibrillar conversion. Our current findings are consistent with preliminary data in which we have shown a seeding effect of fibrils formed from D76N or the natural truncated form of β2-microglobulin lacking the first six N-terminal residues. Interestingly, the hybrid wild-type/variant fibrillar material acquired a thermodynamic stability similar to that of homogenous D76N β2-microglobulin fibrils and significantly higher than the wild-type homogeneous fibrils prepared at neutral pH in the presence of 20% trifluoroethanol. These results suggest that the surface of D76N β2-microglobulin fibrils can favor the transition of the wild-type protein into an amyloid conformation leading to a rapid integration into fibrils. The chaperone crystallin, which is a mild modulator of the lag phase of the variant fibrillogenesis, potently inhibits fibril elongation of the wild-type even once it is absorbed on D76N β2-microglobulin fibrils.
Keywords: Fourier transform IR (FTIR); amyloid; fibril; protein aggregation; protein misfolding; β2-microglobulin.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures



References
-
- Bellotti V., and Chiti F. (2008) Amyloidogenesis in its biological environment: challenging a fundamental issue in protein misfolding diseases. Curr. Opin. Struct. Biol. 18, 771–779 - PubMed
-
- Booth D. R., Sunde M., Bellotti V., Robinson C. V., Hutchinson W. L., Fraser P. E., Hawkins P. N., Dobson C. M., Radford S. E., Blake C. C., and Pepys M. B. (1997) Instability, unfolding and aggregation of human lysozyme variants underlying amyloid fibrillogenesis. Nature 385, 787–793 - PubMed
-
- Colon W., and Kelly J. W. (1992) Partial denaturation of transthyretin is sufficient for amyloid fibril formation in vitro. Biochemistry 31, 8654–8660 - PubMed
-
- Naiki H., Hashimoto N., Suzuki S., Kimura H., Nakakuki K., and Gejyo F. (1997) Establishment of a kinetic model of dialysis-related amyloid fibril extension in vitro. Amyloid 4, 223–232
-
- Valleix S., Gillmore J. D., Bridoux F., Mangione P. P., Dogan A., Nedelec B., Boimard M., Touchard G., Goujon J. M., Lacombe C., Lozeron P., Adams D., Lacroix C., Maisonobe T., Planté-Bordeneuve V., et al. (2012) Hereditary systemic amyloidosis due to Asp76Asn variant β2-microglobulin. N. Engl. J. Med. 366, 2276–2283 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

