Extracellular Adenosine Production by ecto-5'-Nucleotidase (CD73) Enhances Radiation-Induced Lung Fibrosis
- PMID: 26921334
- PMCID: PMC4960984
- DOI: 10.1158/0008-5472.CAN-15-2310
Extracellular Adenosine Production by ecto-5'-Nucleotidase (CD73) Enhances Radiation-Induced Lung Fibrosis
Abstract
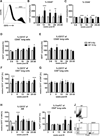
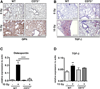
Radiation-induced pulmonary fibrosis is a severe side effect of thoracic irradiation, but its pathogenesis remains poorly understood and no effective treatment is available. In this study, we investigated the role of the extracellular adenosine as generated by the ecto-5'-nucleotidase CD73 in fibrosis development after thoracic irradiation. Exposure of wild-type C57BL/6 mice to a single dose (15 Gray) of whole thorax irradiation triggered a progressive increase in CD73 activity in the lung between 3 and 30 weeks postirradiation. In parallel, adenosine levels in bronchoalveolar lavage fluid (BALF) were increased by approximately 3-fold. Histologic evidence of lung fibrosis was observed by 25 weeks after irradiation. Conversely, CD73-deficient mice failed to accumulate adenosine in BALF and exhibited significantly less radiation-induced lung fibrosis (P < 0.010). Furthermore, treatment of wild-type mice with pegylated adenosine deaminase or CD73 antibodies also significantly reduced radiation-induced lung fibrosis. Taken together, our findings demonstrate that CD73 potentiates radiation-induced lung fibrosis, suggesting that existing pharmacologic strategies for modulating adenosine may be effective in limiting lung toxicities associated with the treatment of thoracic malignancies. Cancer Res; 76(10); 3045-56. ©2016 AACR.
©2016 American Association for Cancer Research.
Conflict of interest statement
No conflict of interest does exist for any of the authors
Figures




References
-
- Graves PR, Siddiqui F, Anscher MS, Movsas B. Radiation pulmonary toxicity: from mechanisms to management. Semin Radiat Oncol. 2010;20:201–207. - PubMed
-
- Kelsey CR, Horwitz ME, Chino JP, Craciunescu O, Steffey B, Folz RJ, et al. Severe pulmonary toxicity after myeloablative conditioning using total body irradiation: an assessment of risk factors. International journal of radiation oncology, biology, physics. 2011;81:812–818. - PubMed
-
- Kasper M, Haroske G. Alterations in the alveolar epithelium after injury leading to pulmonary fibrosis. Histol Histopathol. 1996;11:463–483. - PubMed
-
- Tsoutsou PG, Koukourakis MI. Radiation pneumonitis and fibrosis: mechanisms underlying its pathogenesis and implications for future research. International journal of radiation oncology, biology, physics. 2006;66:1281–1293. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

