Packaging of Campylobacter jejuni into Multilamellar Bodies by the Ciliate Tetrahymena pyriformis
- PMID: 26921427
- PMCID: PMC4836424
- DOI: 10.1128/AEM.03921-15
Packaging of Campylobacter jejuni into Multilamellar Bodies by the Ciliate Tetrahymena pyriformis
Abstract
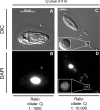
Campylobacter jejuniis the leading cause of bacterial gastroenteritis worldwide. Transmission to humans occurs through consumption of contaminated food or water. The conditions affecting the persistence of C. jejuniin the environment are poorly understood. Some protozoa package and excrete bacteria into multilamellar bodies (MLBs). Packaged bacteria are protected from deleterious conditions, which increases their survival. We hypothesized that C. jejuni could be packaged under aerobic conditions by the amoeba Acanthamoeba castellanii or the ciliate Tetrahymena pyriformis, both of which are able to package other pathogenic bacteria.A. castellanii did not produce MLBs containing C. jejuni In contrast, when incubated with T. pyriformis,C. jejuni was ingested, packaged in MLBs, and then expelled into the milieu. The viability of the bacteria inside MLBs was confirmed by microscopic analyses. The kinetics of C. jejuni culturability showed that packaging increased the survival of C. jejuniup to 60 h, in contrast to the strong survival defect seen in ciliate-free culture. This study suggests that T. pyriformis may increase the risk of persistence of C. jejuniin the environment and its possible transmission between different reservoirs in food and potable water through packaging.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures




Similar articles
-
Survival of Campylobacter jejuni in waterborne protozoa.Appl Environ Microbiol. 2005 Sep;71(9):5560-71. doi: 10.1128/AEM.71.9.5560-5571.2005. Appl Environ Microbiol. 2005. PMID: 16151149 Free PMC article.
-
Survival of Campylobacter jejuni in co-culture with Acanthamoeba castellanii: role of amoeba-mediated depletion of dissolved oxygen.Environ Microbiol. 2012 Aug;14(8):2034-47. doi: 10.1111/j.1462-2920.2011.02655.x. Epub 2011 Dec 19. Environ Microbiol. 2012. PMID: 22176643
-
Variation in Campylobacter jejuni culturability in presence of Acanthamoeba castellanii Neff.Exp Parasitol. 2017 Dec;183:178-181. doi: 10.1016/j.exppara.2017.09.005. Epub 2017 Sep 12. Exp Parasitol. 2017. PMID: 28916459
-
Role of environmental survival in transmission of Campylobacter jejuni.FEMS Microbiol Lett. 2014 Jul;356(1):8-19. doi: 10.1111/1574-6968.12488. Epub 2014 Jun 19. FEMS Microbiol Lett. 2014. PMID: 24888326 Review.
-
Campylobacter-Acanthamoeba interactions.Microbiology (Reading). 2015 May;161(Pt 5):933-947. doi: 10.1099/mic.0.000075. Epub 2015 Mar 10. Microbiology (Reading). 2015. PMID: 25757600 Review.
Cited by
-
Microbial warfare in the wild-the impact of protists on the evolution and virulence of bacterial pathogens.Int Microbiol. 2021 Nov;24(4):559-571. doi: 10.1007/s10123-021-00192-y. Epub 2021 Aug 8. Int Microbiol. 2021. PMID: 34365574 Review.
-
Enhanced transport of bacteria along root systems by protists can impact plant health.Appl Environ Microbiol. 2024 Apr 17;90(4):e0201123. doi: 10.1128/aem.02011-23. Epub 2024 Mar 27. Appl Environ Microbiol. 2024. PMID: 38534145 Free PMC article.
-
Phenotypic and Transcriptomic Responses of Campylobacter jejuni Suspended in an Artificial Freshwater Medium.Front Microbiol. 2017 Sep 20;8:1781. doi: 10.3389/fmicb.2017.01781. eCollection 2017. Front Microbiol. 2017. PMID: 28979243 Free PMC article.
-
Unravelling the importance of the eukaryotic and bacterial communities and their relationship with Legionella spp. ecology in cooling towers: a complex network.Microbiome. 2020 Nov 12;8(1):157. doi: 10.1186/s40168-020-00926-6. Microbiome. 2020. PMID: 33183356 Free PMC article.
-
The dialogue between protozoa and bacteria in a microfluidic device.PLoS One. 2019 Oct 9;14(10):e0222484. doi: 10.1371/journal.pone.0222484. eCollection 2019. PLoS One. 2019. PMID: 31596855 Free PMC article.
References
-
- Sahin O, Fitzgerald C, Stroika S, Zhao S, Sippy RJ, Kwan P, Plummer PJ, Han J, Yaeger MJ, Zhang Q. 2012. Molecular evidence for zoonotic transmission of an emergent, highly pathogenic Campylobacter jejuni clone in the United States. J Clin Microbiol 50:680–687. doi:10.1128/JCM.06167-11. - DOI - PMC - PubMed
-
- Blaser MJ, Engberg J. 2008. Clinical aspects of Campylobacter jejuni and Campylobacter coli infections, p 99–121. In Nachamkin I, Szymanski CM, Blaser MJ (ed), Campylobacter, 3rd ed ASM Press, Washington, DC.
-
- Koga M, Ang CW, Yuki N, Jacobs BC, Herbrink P, van der Meche FGA, Hirata K, van Doorn PA. 2001. Comparative study of preceding Campylobacter jejuni infection in Guillain-Barré syndrome in Japan and the Netherlands. J Neurol Neurosurg Psychiatry 70:693–695. doi:10.1136/jnnp.70.5.693. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

