In-vivo evidence that high mobility group box 1 exerts deleterious effects in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model and Parkinson's disease which can be attenuated by glycyrrhizin
- PMID: 26921471
- PMCID: PMC4867789
- DOI: 10.1016/j.nbd.2016.02.018
In-vivo evidence that high mobility group box 1 exerts deleterious effects in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model and Parkinson's disease which can be attenuated by glycyrrhizin
Abstract
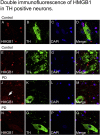
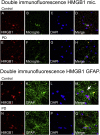
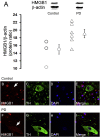
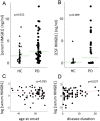
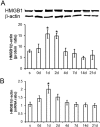
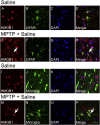
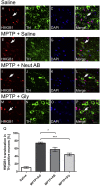
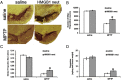
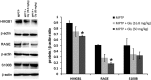
High-mobility group box 1 (HMGB1) is a nuclear and cytosolic protein that is released during tissue damage from immune and non-immune cells - including microglia and neurons. HMGB1 can contribute to progression of numerous chronic inflammatory and autoimmune diseases which is mediated in part by interaction with the receptor for advanced glycation endproducts (RAGE). There is increasing evidence from in vitro studies that HMGB1 may link the two main pathophysiological components of Parkinson's disease (PD), i.e. progressive dopaminergic degeneration and chronic neuroinflammation which underlie the mechanistic basis of PD progression. Analysis of tissue and biofluid samples from PD patients, showed increased HMGB1 levels in human postmortem substantia nigra specimens as well as in the cerebrospinal fluid and serum of PD patients. In a mouse model of PD induced by sub-acute administration of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), systemic administration of neutralizing antibodies to HMGB1 partly inhibited the dopaminergic cell death, and reduced the increase of RAGE and tumour necrosis factor-alpha. The small natural molecule glycyrrhizin, a component from liquorice root which can directly bind to HMGB1, both suppressed MPTP-induced HMGB1 and RAGE upregulation while reducing MPTP-induced dopaminergic cell death in a dose dependent manner. These results provide first in vivo evidence that HMGB1 serves as a powerful bridge between progressive dopaminergic neurodegeneration and chronic neuroinflammation in a model of PD, suggesting that HMGB1 is a suitable target for neuroprotective trials in PD.
Keywords: High-mobility group box 1; MPTP; Parkinson's disease; receptor for advanced glycation endproducts.
Copyright © 2016 The Authors. Published by Elsevier Inc. All rights reserved.
Figures
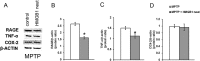
Similar articles
-
Co-treatment with natural HMGB1 inhibitor Glycyrrhizin exerts neuroprotection and reverses Parkinson's disease like pathology in Zebrafish.J Ethnopharmacol. 2022 Jun 28;292:115234. doi: 10.1016/j.jep.2022.115234. Epub 2022 Mar 28. J Ethnopharmacol. 2022. PMID: 35358621
-
Taurochenodeoxycholic acid activates autophagy and suppresses inflammatory responses in microglia of MPTP-induced Parkinson's disease mice via AMPK/mTOR, AKT/NFκB and Pink1/Parkin signaling pathways mediated by Takeda G protein-coupled receptor 5.Free Radic Biol Med. 2025 Aug 1;235:347-363. doi: 10.1016/j.freeradbiomed.2025.04.053. Epub 2025 May 3. Free Radic Biol Med. 2025. PMID: 40324640
-
HMGB1 A box protects neurons by potently inhibiting both microglia and T cell-mediated inflammation in a mouse Parkinson's disease model.Clin Sci (Lond). 2020 Aug 14;134(15):2075-2090. doi: 10.1042/CS20200553. Clin Sci (Lond). 2020. PMID: 32706028
-
The intranasal administration of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP): a new rodent model to test palliative and neuroprotective agents for Parkinson's disease.Curr Pharm Des. 2011;17(5):489-507. doi: 10.2174/138161211795164095. Curr Pharm Des. 2011. PMID: 21375482 Review.
-
High-mobility group box 1 in Parkinson's disease: from pathogenesis to therapeutic approaches.J Neurochem. 2018 Aug;146(3):211-218. doi: 10.1111/jnc.14450. Epub 2018 Jul 3. J Neurochem. 2018. PMID: 29676481 Review.
Cited by
-
Glycyrrhizin Protects Mice Against Experimental Autoimmune Encephalomyelitis by Inhibiting High-Mobility Group Box 1 (HMGB1) Expression and Neuronal HMGB1 Release.Front Immunol. 2018 Jul 2;9:1518. doi: 10.3389/fimmu.2018.01518. eCollection 2018. Front Immunol. 2018. PMID: 30013568 Free PMC article.
-
High-sensitivity C-reactive protein and high mobility group box-1 levels in Parkinson's disease.Neurol Sci. 2019 Jan;40(1):167-173. doi: 10.1007/s10072-018-3611-z. Epub 2018 Oct 23. Neurol Sci. 2019. PMID: 30353300
-
Detrimental Effects of HMGB-1 Require Microglial-Astroglial Interaction: Implications for the Status Epilepticus -Induced Neuroinflammation.Front Cell Neurosci. 2019 Aug 27;13:380. doi: 10.3389/fncel.2019.00380. eCollection 2019. Front Cell Neurosci. 2019. PMID: 31507379 Free PMC article.
-
High Mobility Group Box 1 (HMGB1): Potential Target in Sepsis-Associated Encephalopathy.Cells. 2023 Apr 4;12(7):1088. doi: 10.3390/cells12071088. Cells. 2023. PMID: 37048161 Free PMC article. Review.
-
Role and Therapeutic Potential of RAGE Signaling in Neurodegeneration.Curr Drug Targets. 2022;23(12):1191-1209. doi: 10.2174/1389450123666220610171005. Curr Drug Targets. 2022. PMID: 35702767 Free PMC article.
References
-
- Andersson A., Covacu R., Sunnemark D., Danilov A.I., Dal Bianco A., Khademi M., Wallstrom E., Lobell A., Brundin L., Lassmann H., Harris R.A. Pivotal advance: HMGB1 expression in active lesions of human and experimental multiple sclerosis. J. Leukoc. Biol. 2008;84:1248–1255. - PubMed
-
- Bustin M., Reeves R. High-mobility-group chromosomal proteins: architectural components that facilitate chromatin function. Prog. Nucleic Acid Res. Mol. Biol. 1996;54:35–100. - PubMed
-
- de la Fuente-Fernández R., Schulzer M., Kuramoto L., Cragg J., Ramachandiran N., Au W.L., Mak E., McKenzie J., McCormick S., Sossi V. Age-specific progression of nigrostriatal dysfunction in Parkinson's disease. Ann. Neurol. 2011;69:803–810. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

