Eliminating microglia in Alzheimer's mice prevents neuronal loss without modulating amyloid-β pathology
- PMID: 26921617
- PMCID: PMC5006229
- DOI: 10.1093/brain/aww016
Eliminating microglia in Alzheimer's mice prevents neuronal loss without modulating amyloid-β pathology
Abstract
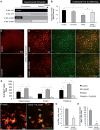
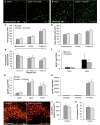
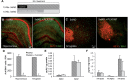
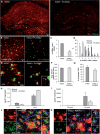
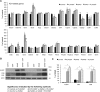
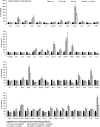
In addition to amyloid-β plaque and tau neurofibrillary tangle deposition, neuroinflammation is considered a key feature of Alzheimer's disease pathology. Inflammation in Alzheimer's disease is characterized by the presence of reactive astrocytes and activated microglia surrounding amyloid plaques, implicating their role in disease pathogenesis. Microglia in the healthy adult mouse depend on colony-stimulating factor 1 receptor (CSF1R) signalling for survival, and pharmacological inhibition of this receptor results in rapid elimination of nearly all of the microglia in the central nervous system. In this study, we set out to determine if chronically activated microglia in the Alzheimer's disease brain are also dependent on CSF1R signalling, and if so, how these cells contribute to disease pathogenesis. Ten-month-old 5xfAD mice were treated with a selective CSF1R inhibitor for 1 month, resulting in the elimination of ∼80% of microglia. Chronic microglial elimination does not alter amyloid-β levels or plaque load; however, it does rescue dendritic spine loss and prevent neuronal loss in 5xfAD mice, as well as reduce overall neuroinflammation. Importantly, behavioural testing revealed improvements in contextual memory. Collectively, these results demonstrate that microglia contribute to neuronal loss, as well as memory impairments in 5xfAD mice, but do not mediate or protect from amyloid pathology.
Keywords: Alzheimer’s disease; amyloid; cognition; inflammation; microglia.
© The Author (2016). Published by Oxford University Press on behalf of the Guarantors of Brain. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures



References
-
- Akama KT, Van Eldik LJ.Beta-amyloid stimulation of inducible nitric-oxide synthase in astrocytes is interleukin-1beta- and tumor necrosis factor-alpha (TNFalpha)-dependent, and involves a TNFalpha receptor-associated factor- and NFkappaB-inducing kinase-dependent signaling mechanism. J Biol Chem 2000; 275: 7918–24. - PubMed
-
- Akiyama H, Arai T, Kondo H, Tanno E, Haga C, Ikeda K. Cell mediators of inflammation in the Alzheimer disease brain. Alzheimer Dis Assoc Disord 2000; 14 (Suppl 1): S47–53. - PubMed
-
- Ando K, Brion JP, Stygelbout V, Suain V, Authelet M, Dedecker R, et al. Clathrin adaptor CALM/PICALM is associated with neurofibrillary tangles and is cleaved in Alzheimer's brains. Acta Neuropathol 2013; 125: 861–78. - PubMed
-
- Blinzinger K, Kreutzberg G. Displacement of synaptic terminals from regenerating motoneurons by microglial cells. Z Zellforsch Mikrosk Anat 1968; 85: 145–57. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

