Acetaminophen from liver to brain: New insights into drug pharmacological action and toxicity
- PMID: 26921661
- PMCID: PMC4912877
- DOI: 10.1016/j.phrs.2016.02.020
Acetaminophen from liver to brain: New insights into drug pharmacological action and toxicity
Abstract
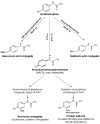
Acetaminophen (APAP) is a well-known analgesic and antipyretic drug. It is considered to be safe when administered within its therapeutic range, but in cases of acute intoxication, hepatotoxicity can occur. APAP overdose is the leading cause of acute liver failure in the northern hemisphere. Historically, studies on APAP toxicity have been focused on liver, with alterations in brain function attributed to secondary effects of acute liver failure. However, in the last decade the pharmacological mechanism of APAP as a cannabinoid system modulator has been documented and some articles have reported "in situ" toxicity by APAP in brain tissue at high doses. Paradoxically, low doses of APAP have been reported to produce the opposite, neuroprotective effects. In this paper we present a comprehensive, up-to-date overview of hepatic toxicity as well as a thorough review of both toxic and beneficial effects of APAP in brain.
Keywords: Acetaminophen; Caspase; DAMPs; Hepatic toxicity; Necroptosis; trpv1.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Figures


References
-
- Morse HN. Ueber eine neue Darstellungsmethode der Acetylamidophenole. Ber Deutscher Chem Ges. 1878;11:232–233.
-
- Von Mering J. Beitrage zur Kenntniss der Antipyretica. Ther Monatsch. 1893;7:577–587.
-
- Brodie BB, AXELROD J. The fate of acetanilide in man. J Pharmacol Exp Ther. 1948;94:29–38. - PubMed
-
- Pandolfini C, Bonati M. A literature review on off-label drug use in children. Eur J Pediatr. 2005;164:552–558. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

