Lysosomes in cancer-living on the edge (of the cell)
- PMID: 26921697
- PMCID: PMC7611282
- DOI: 10.1016/j.ceb.2016.02.009
Lysosomes in cancer-living on the edge (of the cell)
Abstract
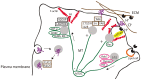
The lysosomes have definitely polished their status inside the cell. Being discovered as the last resort of discarded cellular biomass, the steady rising of this versatile signaling organelle is currently ongoing. This review discusses the recent data on the unconventional functions of lysosomes, focusing mainly on the less studied lysosomes residing in the cellular periphery. We emphasize our discussion on the emerging paths the lysosomes have taken in promoting cancer progression to metastatic disease. Finally, we address how the altered cancerous lysosomes in metastatic cancers may be specifically targeted and what are the pending questions awaiting for elucidation.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Figures

References
-
- de Duve C. The lysosome turns fifty. Nat Cell Biol. 2005;7:847–849. - PubMed
-
- Kallunki T, Olsen OD, Jaattela M. Cancer-associated lysosomal changes: friends or foes? Oncogene. 2013;32:1995–2004. - PubMed
-
- Eskelinen EL, Tanaka Y, Saftig P. At the acidic edge: emerging functions for lysosomal membrane proteins. Trends Cell Biol. 2003;13:137–145. - PubMed
-
- Appelqvist H, Waster P, Kagedal K, Ollinger K. The lysosome: from waste bag to potential therapeutic target. JMol Cell Biol. 2013;5:214–226. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

