Integration of inhibitory and excitatory effects of α7 nicotinic acetylcholine receptor activation in the prelimbic cortex regulates network activity and plasticity
- PMID: 26921769
- PMCID: PMC4881417
- DOI: 10.1016/j.neuropharm.2016.02.028
Integration of inhibitory and excitatory effects of α7 nicotinic acetylcholine receptor activation in the prelimbic cortex regulates network activity and plasticity
Abstract
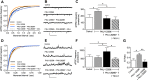
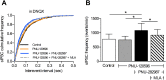
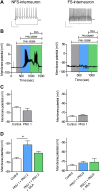
Cognitive and attentional processes governed by the prefrontal cortex (PFC) are influenced by cholinergic innervation. Here we have explored the role of α7 nicotinic acetylcholine receptors (nAChRs) as mediators of cholinergic signalling in the dorsomedial (prelimbic) PFC, using mouse brain slice electrophysiology. Activation of α7 nAChRs located on glutamatergic terminals and cell soma of GABAergic interneurons increased excitation and inhibition, respectively, in layer V of the prelimbic cortex. These actions were distinguished by their differential dependence on local acetylcholine (ACh): potentiation of endogenous cholinergic signalling with the positive allosteric modulator, PNU-120596, enhanced spontaneous excitatory events, an effect that was further increased by inhibition of acetylcholinesterase. In contrast, α7 nicotinic modulation of inhibitory signalling required addition of exogenous agonist (PNU-282987) as well as PNU-120596, and was unaffected by acetylcholinesterase inhibition. Thus α7 nAChRs can bi-directionally regulate network activity in the prelimbic cortex, depending on the magnitude and localisation of cholinergic signalling. This bidirectional influence is manifest in dual effects of α7 nAChRs on theta-burst-induced long-term potentiation (LTP) in layer V of the prelimbic cortex. Antagonism of α7 nAChRs significantly decreased LTP implicating a contribution from endogenous ACh, consistent with the ability of local ACh to enhance glutamatergic signalling. Exogenous agonist plus potentiator also decreased LTP, indicative of the influence of this drug combination on inhibitory signalling. Thus α7 nAChRs make a complex contribution to network activity and synaptic plasticity in the prelimbic cortex.
Keywords: GABA; Glutamate; LTP; Nicotinic receptors; Prefrontal cortex; Prelimbic cortex.
Copyright © 2016 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures




References
-
- Alkondon M., Pereira E.F., Barbosa C.T., Albuquerque E.X. Neuronal nicotinic acetylcholine receptor activation modulates gamma-aminobutyric acid release from CA1 neurons of rat hippocampal slices. J. Pharmacol. Exp. Ther. 1997;283:1396–1411. - PubMed
-
- Aracri P., Consonni S., Morini R., Perrella M., Rodighiero S., Amadeo A., Becchetti A. Tonic modulation of GABA release by nicotinic acetylcholine receptors in layer V of the murine prefrontal cortex. Cereb. Cortex. 2010;20:1539–1555. - PubMed
-
- Aston-Jones G., Shaver R., Dinan T.G. Nucleus basalis neurons exhibit axonal branching with decreased impulse conduction velocity in rat cerebrocortex. Brain Res. 1985;325:271–285. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

