Isothiocyanates as effective agents against enterohemorrhagic Escherichia coli: insight to the mode of action
- PMID: 26922906
- PMCID: PMC4770323
- DOI: 10.1038/srep22263
Isothiocyanates as effective agents against enterohemorrhagic Escherichia coli: insight to the mode of action
Abstract
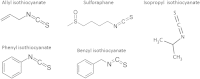
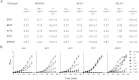
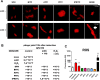
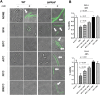
Production of Shiga toxins by enterohemorrhagic Escherichia coli (EHEC) which is responsible for the pathogenicity of these strains, is strictly correlated with induction of lambdoid bacteriophages present in the host's genome, replication of phage DNA and expression of stx genes. Antibiotic treatment of EHEC infection may lead to induction of prophage into a lytic development, thus increasing the risk of severe complications. This, together with the spread of multi-drug resistance, increases the need for novel antimicrobial agents. We report here that isothiocyanates (ITC), plant secondary metabolites, such as sulforaphane (SFN), allyl isothiocyanate (AITC), benzyl isothiocynanate (BITC), phenyl isothiocyanate (PITC) and isopropyl isothiocyanate (IPRITC), inhibit bacterial growth and lytic development of stx-harboring prophages. The mechanism underlying the antimicrobial effect of ITCs involves the induction of global bacterial stress regulatory system, the stringent response. Its alarmone, guanosine penta/tetraphosphate ((p)ppGpp) affects major cellular processes, including nucleic acids synthesis, which leads to the efficient inhibition of both, prophage induction and toxin synthesis, abolishing in this way EHEC virulence for human and simian cells. Thus, ITCs could be considered as potential therapeutic agents in EHEC infections.
Figures


References
-
- Beutin L. & Martin A. Outbreak of Shiga toxin-producing Escherichia coli (STEC) O104:H4 infection in Germany causes a paradigm shift with regard to human pathogenicity of STEC strains. J. Food Prot. 75, 408–418 (2012). - PubMed
-
- Bloch S. A., Felczykowska A. & Nejman-Faleńczyk B. Escherichia coli O104:H4 outbreak-have we learnt a lesson from it? Acta Biochim. Pol. 59, 483–488 (2012). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

