A new paramagnetically shifted imaging probe for MRI
- PMID: 26922918
- PMCID: PMC5324534
- DOI: 10.1002/mrm.26185
A new paramagnetically shifted imaging probe for MRI
Abstract
Purpose: To develop and characterize a new paramagnetic contrast agent for molecular imaging by MRI.
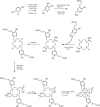
Methods: A contrast agent was developed for direct MRI detection through the paramagnetically shifted proton magnetic resonances of two chemically equivalent tert-butyl reporter groups within a dysprosium(III) complex. The complex was characterized in phantoms and imaged in physiologically intact mice at 7 Tesla (T) using three-dimensional (3D) gradient echo and spectroscopic imaging (MRSI) sequences to measure spatial distribution and signal frequency.
Results: The reporter protons reside ∼6.5 Å from the paramagnetic center, resulting in fast T1 relaxation (T1 = 8 ms) and a large paramagnetic frequency shift exceeding 60 ppm. Fast relaxation allowed short scan repetition times with high excitation flip angle, resulting in high sensitivity. The large dipolar shift allowed direct frequency selective excitation and acquisition of the dysprosium(III) complex, independent of the tissue water signal. The biokinetics of the complex were followed in vivo with a temporal resolution of 62 s following a single, low-dose intravenous injection. The lower concentration limit for detection was ∼23 μM. Through MRSI, the temperature dependence of the paramagnetic shift (0.28 ppm.K-1 ) was exploited to examine tissue temperature variation.
Conclusions: These data demonstrate a new MRI agent with the potential for physiological monitoring by MRI. Magn Reson Med 77:1307-1317, 2017. © 2016 The Authors Magnetic Resonance in Medicine published by Wiley Periodicals, Inc. on behalf of International Society for Magnetic Resonance in Medicine. This is an open access article under the terms of the Creative Commons Attribution License, which permits use, distribution and reproduction in any medium, provided the original work is properly cited.
Keywords: contrast agent; molecular imaging; paramagnetic shift; temperature mapping.
© 2016 The Authors Magnetic Resonance in Medicine published by Wiley Periodicals, Inc. on behalf of International Society for Magnetic Resonance in Medicine.
Figures






References
-
- Caravan P, Ellison JJ, McMurry TJ, Lauffer RB. Gadolinium(III) chelates as MRI contrast agents: structure, dynamics, and applications. Chem Rev 1999;99:2293–2352. - PubMed
-
- Chen HH, Wei L, Rotile NJ, Farrar CT, Tanabe KK, Fuchs BC, Caravan P. Quantitative molecular magnetic resonance imaging (MRI) of lysyl oxidase‐mediated collagen crosslinking during liver fibrosis. Hepatology 2014;60:205A.
-
- Sibson NR, Blamire AM, Bernades‐Silva M, Laurent S, Boutry S, Muller RN, Styles P, Anthony DC. MRI detection of early endothelial activation in brain inflammation. Magn Reson Med 2004;51:248–252. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

