Dynamic Protein Interaction Networks and New Structural Paradigms in Signaling
- PMID: 26922996
- PMCID: PMC5342629
- DOI: 10.1021/acs.chemrev.5b00548
Dynamic Protein Interaction Networks and New Structural Paradigms in Signaling
Abstract
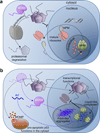
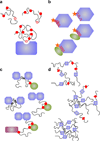
Understanding signaling and other complex biological processes requires elucidating the critical roles of intrinsically disordered proteins (IDPs) and regions (IDRs), which represent ∼30% of the proteome and enable unique regulatory mechanisms. In this review, we describe the structural heterogeneity of disordered proteins that underpins these mechanisms and the latest progress in obtaining structural descriptions of conformational ensembles of disordered proteins that are needed for linking structure and dynamics to function. We describe the diverse interactions of IDPs that can have unusual characteristics such as "ultrasensitivity" and "regulated folding and unfolding". We also summarize the mounting data showing that large-scale assembly and protein phase separation occurs within a variety of signaling complexes and cellular structures. In addition, we discuss efforts to therapeutically target disordered proteins with small molecules. Overall, we interpret the remodeling of disordered state ensembles due to binding and post-translational modifications within an expanded framework for allostery that provides significant insights into how disordered proteins transmit biological information.
Figures

References
-
- Frauenfelder H, Sligar SG, Wolynes PG. The Energy Landscapes and Motions of Proteins. Science. 1991;254:1598–1603. - PubMed
-
- Biehl R, Richter D. Slow Internal Protein Dynamics in Solution. J. Phys. Condens. Matter. 2014;26:503103. - PubMed
-
- Tompa P. Intrinsically Disordered Proteins: A 10-Year Recap. Trends Biochem. Sci. 2012;37:509–516. - PubMed
-
- Cohen P. The Regulation of Protein Function by Multisite Phosphorylation--a 25 Year Update. Trends Biochem. Sci. 2000;25:596–601. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

