Disclosure of amyloid status is not a barrier to recruitment in preclinical Alzheimer's disease clinical trials
- PMID: 26923411
- PMCID: PMC4773920
- DOI: 10.1016/j.neurobiolaging.2015.11.007
Disclosure of amyloid status is not a barrier to recruitment in preclinical Alzheimer's disease clinical trials
Abstract
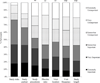
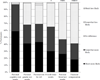
Preclinical Alzheimer's disease (AD) clinical trials may require participants to learn if they meet biomarker enrollment criteria. To examine whether this requirement will impact trial recruitment, we presented 132 older community volunteers who self-reported normal cognition with 1 of 2 hypothetical informed consent forms (ICFs) describing an AD prevention clinical trial. Both ICFs described amyloid Positron Emission Tomography scans. One ICF stated that scan results would not be shared with the participants (blinded enrollment); the other stated that only persons with elevated amyloid would be eligible (transparent enrollment). Participants rated their likelihood of enrollment and completed an interview with a research assistant. We found no difference between the groups in willingness to participate. Study risks and the requirement of a study partner were reported as the most important factors in the decision whether to enroll. The requirement of biomarker disclosure may not slow recruitment to preclinical AD trials.
Keywords: Biomarker; Disclosure; Preclinical Alzheimer's disease; Recruitment.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures
References
-
- Washington (DC): Workshop Summary; 2014. Issues in Returning Individual Results from Genome Research Using Population-Based Banked Specimens, with a Focus on the National Health and Nutrition Examination Survey. - PubMed
-
- Burns JM, Klunk WE. Predicting positivity for a new era of Alzheimer disease prevention trials. Neurology. 2012;79(15):1530–1531. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

