Fatty Acid Oxidation-Driven Src Links Mitochondrial Energy Reprogramming and Oncogenic Properties in Triple-Negative Breast Cancer
- PMID: 26923594
- PMCID: PMC4809061
- DOI: 10.1016/j.celrep.2016.02.004
Fatty Acid Oxidation-Driven Src Links Mitochondrial Energy Reprogramming and Oncogenic Properties in Triple-Negative Breast Cancer
Abstract
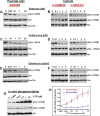
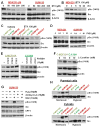
Transmitochondrial cybrids and multiple OMICs approaches were used to understand mitochondrial reprogramming and mitochondria-regulated cancer pathways in triple-negative breast cancer (TNBC). Analysis of cybrids and established breast cancer (BC) cell lines showed that metastatic TNBC maintains high levels of ATP through fatty acid β oxidation (FAO) and activates Src oncoprotein through autophosphorylation at Y419. Manipulation of FAO including the knocking down of carnitine palmitoyltransferase-1A (CPT1) and 2 (CPT2), the rate-limiting proteins of FAO, and analysis of patient-derived xenograft models confirmed the role of mitochondrial FAO in Src activation and metastasis. Analysis of TCGA and other independent BC clinical data further reaffirmed the role of mitochondrial FAO and CPT genes in Src regulation and their significance in BC metastasis.
Copyright © 2016 The Authors. Published by Elsevier Inc. All rights reserved.
Figures





References
-
- Acin-Perez R, Carrascoso I, Baixauli F, Roche-Molina M, Latorre-Pellicer A, Fernandez-Silva P, Mittelbrunn M, Sanchez-Madrid F, Perez-Martos A, Lowell CA, et al. ROS-triggered phosphorylation of complex II by Fgr kinase regulates cellular adaptation to fuel use. Cell Metab. 2014;19:1020–1033. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- NIAID P30AI036211/PHS HHS/United States
- R01 CA112305/CA/NCI NIH HHS/United States
- R21 CA173150/CA/NCI NIH HHS/United States
- P01CA30195/CA/NCI NIH HHS/United States
- R21CA179720S1/CA/NCI NIH HHS/United States
- U01CA179674/CA/NCI NIH HHS/United States
- R21CA185516/CA/NCI NIH HHS/United States
- U01 CA179674/CA/NCI NIH HHS/United States
- R21CA179720/CA/NCI NIH HHS/United States
- R21 CA185516/CA/NCI NIH HHS/United States
- R21CA173150/CA/NCI NIH HHS/United States
- U54-CA149196/CA/NCI NIH HHS/United States
- R01CA112305/CA/NCI NIH HHS/United States
- P30 CA125123/CA/NCI NIH HHS/United States
- R01 CA184208/CA/NCI NIH HHS/United States
- NIH-R01CA184208/CA/NCI NIH HHS/United States
- P50CA50183/CA/NCI NIH HHS/United States
- P01 CA030195/CA/NCI NIH HHS/United States
- NCRR S10RR024574/PHS HHS/United States
- P30 AI036211/AI/NIAID NIH HHS/United States
- S10 RR024574/RR/NCRR NIH HHS/United States
- U54 CA149196/CA/NCI NIH HHS/United States
- R21CA179720S2/CA/NCI NIH HHS/United States
- R21 CA179720/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

