Reciprocal Degradation of YME1L and OMA1 Adapts Mitochondrial Proteolytic Activity during Stress
- PMID: 26923599
- PMCID: PMC4785047
- DOI: 10.1016/j.celrep.2016.02.011
Reciprocal Degradation of YME1L and OMA1 Adapts Mitochondrial Proteolytic Activity during Stress
Abstract
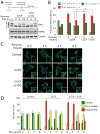
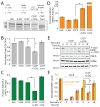
The mitochondrial inner membrane proteases YME1L and OMA1 are critical regulators of essential mitochondrial functions, including inner membrane proteostasis maintenance and mitochondrial dynamics. Here, we show that YME1L and OMA1 are reciprocally degraded in response to distinct types of cellular stress. OMA1 is degraded through a YME1L-dependent mechanism in response to toxic insults that depolarize the mitochondrial membrane. Alternatively, insults that depolarize mitochondria and deplete cellular ATP stabilize active OMA1 and promote YME1L degradation. We show that the differential degradation of YME1L and OMA1 alters their proteolytic processing of the dynamin-like GTPase OPA1, a critical regulator of mitochondrial inner membrane morphology, which influences the recovery of tubular mitochondria following membrane-depolarization-induced fragmentation. Our results reveal the differential stress-induced degradation of YME1L and OMA1 as a mechanism for sensitively adapting mitochondrial inner membrane protease activity and function in response to distinct types of cellular insults.
Copyright © 2016 The Authors. Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Anand R, Langer T, Baker MJ. Proteolytic control of mitochondrial function and morphogenesis. Biochim Biophys Acta. 2013;1833:195–204. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

