Cognitive impairment by antibiotic-induced gut dysbiosis: Analysis of gut microbiota-brain communication
- PMID: 26923630
- PMCID: PMC5014122
- DOI: 10.1016/j.bbi.2016.02.020
Cognitive impairment by antibiotic-induced gut dysbiosis: Analysis of gut microbiota-brain communication
Abstract
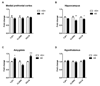
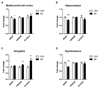
Emerging evidence indicates that disruption of the gut microbial community (dysbiosis) impairs mental health. Germ-free mice and antibiotic-induced gut dysbiosis are two approaches to establish causality in gut microbiota-brain relationships. However, both models have limitations, as germ-free mice display alterations in blood-brain barrier and brain ultrastructure and antibiotics may act directly on the brain. We hypothesized that the concerns related to antibiotic-induced gut dysbiosis can only adequately be addressed if the effect of intragastric treatment of adult mice with multiple antibiotics on (i) gut microbial community, (ii) metabolite profile in the colon, (iii) circulating metabolites, (iv) expression of neuronal signaling molecules in distinct brain areas and (v) cognitive behavior is systematically investigated. Of the antibiotics used (ampicillin, bacitracin, meropenem, neomycin, vancomycin), ampicillin had some oral bioavailability but did not enter the brain. 16S rDNA sequencing confirmed antibiotic-induced microbial community disruption, and metabolomics revealed that gut dysbiosis was associated with depletion of bacteria-derived metabolites in the colon and alterations of lipid species and converted microbe-derived molecules in the plasma. Importantly, novel object recognition, but not spatial, memory was impaired in antibiotic-treated mice. This cognitive deficit was associated with brain region-specific changes in the expression of cognition-relevant signaling molecules, notably brain-derived neurotrophic factor, N-methyl-d-aspartate receptor subunit 2B, serotonin transporter and neuropeptide Y system. We conclude that circulating metabolites and the cerebral neuropeptide Y system play an important role in the cognitive impairment and dysregulation of cerebral signaling molecules due to antibiotic-induced gut dysbiosis.
Keywords: Antibiotic; Brain; Cognition; Dysbiosis; GRIN2B; Gut; Metabolome; Microbiome; Neuropeptide Y; Serotonin transporter.
Copyright © 2016 The Authors. Published by Elsevier Inc. All rights reserved.
Figures

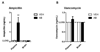



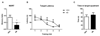

Comment in
-
From gut to brain: Microbiota depletion in mice as a tool to explore causality.Brain Behav Immun. 2021 May;94:4-5. doi: 10.1016/j.bbi.2021.02.029. Epub 2021 Mar 1. Brain Behav Immun. 2021. PMID: 33662505 No abstract available.
References
-
- Bajad SU, Lu W, Kimball EH, Yuan J, Peterson C, Rabinowitz JD. Separation and quantitation of water soluble cellular metabolites by hydrophilic interaction chromatography-tandem mass spectrometry. J Chromatogr A. 2006;1125:76–88. S0021-9673(06)00993-9 [pii] - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

