Super-Resolved Traction Force Microscopy (STFM)
- PMID: 26923775
- PMCID: PMC4898862
- DOI: 10.1021/acs.nanolett.6b00273
Super-Resolved Traction Force Microscopy (STFM)
Abstract
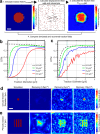
Measuring small forces is a major challenge in cell biology. Here we improve the spatial resolution and accuracy of force reconstruction of the well-established technique of traction force microscopy (TFM) using STED microscopy. The increased spatial resolution of STED-TFM (STFM) allows a greater than 5-fold higher sampling of the forces generated by the cell than conventional TFM, accessing the nano instead of the micron scale. This improvement is highlighted by computer simulations and an activating RBL cell model system.
Keywords: Super-resolution microscopy; actin cytoskeleton; mechanobiology; traction force microscopy.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Trepat X.; Wasserman M. R.; Angelini T. E.; Millet E.; Weitz D. A.; Butler J. P.; Fredberg J. J. Nat. Phys. 2009, 5 (6), 426–430. 10.1038/nphys1269. - DOI
Publication types
MeSH terms
Grants and funding
- MC_UU_12010/9/MRC_/Medical Research Council/United Kingdom
- G0902418/MRC_/Medical Research Council/United Kingdom
- MC_UU_12010/4/MRC_/Medical Research Council/United Kingdom
- BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- MC_UU_12025/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources

