Formin Is Associated with Left-Right Asymmetry in the Pond Snail and the Frog
- PMID: 26923788
- PMCID: PMC4791482
- DOI: 10.1016/j.cub.2015.12.071
Formin Is Associated with Left-Right Asymmetry in the Pond Snail and the Frog
Abstract
While components of the pathway that establishes left-right asymmetry have been identified in diverse animals, from vertebrates to flies, it is striking that the genes involved in the first symmetry-breaking step remain wholly unknown in the most obviously chiral animals, the gastropod snails. Previously, research on snails was used to show that left-right signaling of Nodal, downstream of symmetry breaking, may be an ancestral feature of the Bilateria [1 and 2]. Here, we report that a disabling mutation in one copy of a tandemly duplicated, diaphanous-related formin is perfectly associated with symmetry breaking in the pond snail. This is supported by the observation that an anti-formin drug treatment converts dextral snail embryos to a sinistral phenocopy, and in frogs, drug inhibition or overexpression by microinjection of formin has a chirality-randomizing effect in early (pre-cilia) embryos. Contrary to expectations based on existing models [3, 4 and 5], we discovered asymmetric gene expression in 2- and 4-cell snail embryos, preceding morphological asymmetry. As the formin-actin filament has been shown to be part of an asymmetry-breaking switch in vitro [6 and 7], together these results are consistent with the view that animals with diverse body plans may derive their asymmetries from the same intracellular chiral elements [8].
Copyright © 2016 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures

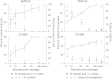
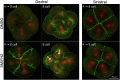

Comment in
-
Snail Chirality: The Unwinding.Curr Biol. 2016 Mar 7;26(5):R215-7. doi: 10.1016/j.cub.2016.02.008. Curr Biol. 2016. PMID: 26954445
References
-
- Hierck B.P., Witte B., Poelmann R.E., Gittenberger-de Groot A.C., Gittenberger E. Chirality in snails is determined by highly conserved asymmetry genes. J. Molluscan Stud. 2005;71:192–195.
-
- Freeman G., Lundelius J.W. Evolutionary implications of the mode of D quadrant specification in coelomates with spiral cleavage. J. Evol. Biol. 1992;5:205–247.
-
- Lambert J.D., Nagy L.M. Asymmetric inheritance of centrosomally localized mRNAs during embryonic cleavages. Nature. 2002;420:682–686. - PubMed
-
- Martindale M.Q. The ‘organizing’ role of the D quadrant in an equal-cleaving spiralian, Lymnaea stagnalis as studied by UV laser deletion of macromeres at intervals between third and fourth quartet formation. International Journal of Invertebrate Reproduction and Development. 1986;9:229–242.
Publication types
MeSH terms
Substances
Grants and funding
- BB/F021135/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- G0900740/MRC_/Medical Research Council/United Kingdom
- U54CA143876/CA/NCI NIH HHS/United States
- BB/F018940/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- G00661X/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- U54 CA143876/CA/NCI NIH HHS/United States
- F021135/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- WT_/Wellcome Trust/United Kingdom
- BB/G00661X/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- MR/K001744/1/MRC_/Medical Research Council/United Kingdom
- WT098051/WT_/Wellcome Trust/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

