Immunotherapy and Novel Combinations in Oncology: Current Landscape, Challenges, and Opportunities
- PMID: 26924066
- PMCID: PMC5351311
- DOI: 10.1111/cts.12391
Immunotherapy and Novel Combinations in Oncology: Current Landscape, Challenges, and Opportunities
Figures
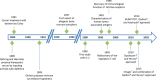
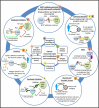
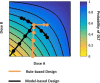
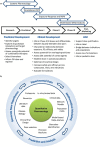
References
-
- Couzin‐Frankel, J. Cancer immunotherapy. Science 342, 1432–1433 (2013). - PubMed
-
- Bray, F. , Jemal, A. , Grey, N. , Ferlay, J. & Forman, D . Global cancer transitions according to the Human Development Index (2008–2030): a population‐based study. Lancet Oncol. 13, 790–801. - PubMed
-
- Chen, D.S. & Mellman, I. Oncology meets immunology: the cancer‐immunity cycle. Immunity 39, 1–10 (2013). - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

