Identification of Meflin as a Potential Marker for Mesenchymal Stromal Cells
- PMID: 26924503
- PMCID: PMC4770287
- DOI: 10.1038/srep22288
Identification of Meflin as a Potential Marker for Mesenchymal Stromal Cells
Abstract
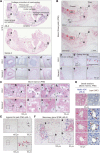
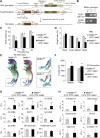
Bone marrow-derived mesenchymal stromal cells (BM-MSCs) in culture are derived from BM stromal cells or skeletal stem cells. Whereas MSCs have been exploited in clinical medicine, the identification of MSC-specific markers has been limited. Here, we report that a cell surface and secreted protein, Meflin, is expressed in cultured MSCs, fibroblasts and pericytes, but not other types of cells including epithelial, endothelial and smooth muscle cells. In vivo, Meflin is expressed by immature osteoblasts and chondroblasts. In addition, Meflin is found on stromal cells distributed throughout the BM, and on pericytes and perivascular cells in multiple organs. Meflin maintains the undifferentiated state of cultured MSCs and is downregulated upon their differentiation, consistent with the observation that Meflin-deficient mice exhibit increased number of osteoblasts and accelerated bone development. In the bone and BM, Meflin is more highly expressed in primitive stromal cells that express platelet-derived growth factor receptor α and Sca-1 than the Sca-1-negative adipo-osteogenic progenitors, which create a niche for hematopoiesis. Those results are consistent with a decrease in the number of clonogenic colony-forming unit-fibroblasts within the BM of Meflin-deficient mice. These preliminary data suggest that Meflin is a potential marker for cultured MSCs and their source cells in vivo.
Figures






Similar articles
-
Meflin defines mesenchymal stem cells and/or their early progenitors with multilineage differentiation capacity.Genes Cells. 2021 Jul;26(7):495-512. doi: 10.1111/gtc.12855. Epub 2021 May 17. Genes Cells. 2021. PMID: 33960573 Free PMC article.
-
Roles of the Mesenchymal Stromal/Stem Cell Marker Meflin in Cardiac Tissue Repair and the Development of Diastolic Dysfunction.Circ Res. 2019 Aug 2;125(4):414-430. doi: 10.1161/CIRCRESAHA.119.314806. Epub 2019 Jun 21. Circ Res. 2019. PMID: 31221024
-
Meflin-Positive Cancer-Associated Fibroblasts Inhibit Pancreatic Carcinogenesis.Cancer Res. 2019 Oct 15;79(20):5367-5381. doi: 10.1158/0008-5472.CAN-19-0454. Epub 2019 Aug 22. Cancer Res. 2019. PMID: 31439548
-
Mesenchymal Stem Cells and Pericytes: To What Extent Are They Related?Stem Cells Dev. 2016 Dec 15;25(24):1843-1852. doi: 10.1089/scd.2016.0109. Epub 2016 Nov 3. Stem Cells Dev. 2016. PMID: 27702398 Review.
-
Roles of the Mesenchymal Stromal/Stem Cell Marker Meflin/Islr in Cancer Fibrosis.Front Cell Dev Biol. 2021 Oct 5;9:749924. doi: 10.3389/fcell.2021.749924. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34676218 Free PMC article. Review.
Cited by
-
Pancreatic ductal adenocarcinoma: tumor microenvironment and problems in the development of novel therapeutic strategies.Clin Exp Med. 2023 Jul;23(3):619-643. doi: 10.1007/s10238-022-00886-1. Epub 2022 Sep 9. Clin Exp Med. 2023. PMID: 36085429 Review.
-
A novel renal perivascular mesenchymal cell subset gives rise to fibroblasts distinct from classic myofibroblasts.Sci Rep. 2022 Mar 30;12(1):5389. doi: 10.1038/s41598-022-09331-5. Sci Rep. 2022. PMID: 35354870 Free PMC article.
-
The Cellular Origins of Cancer-Associated Fibroblasts and Their Opposing Contributions to Pancreatic Cancer Growth.Front Cell Dev Biol. 2021 Sep 27;9:743907. doi: 10.3389/fcell.2021.743907. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34646829 Free PMC article. Review.
-
Heterogeneity of Cancer-Associated Fibroblasts and the Tumor Immune Microenvironment in Pancreatic Cancer.Cancers (Basel). 2022 Aug 18;14(16):3994. doi: 10.3390/cancers14163994. Cancers (Basel). 2022. PMID: 36010986 Free PMC article. Review.
-
Apoptosis in the Pancreatic Cancer Tumor Microenvironment-The Double-Edged Sword of Cancer-Associated Fibroblasts.Cells. 2021 Jul 1;10(7):1653. doi: 10.3390/cells10071653. Cells. 2021. PMID: 34359823 Free PMC article. Review.
References
-
- Friedenstein A. J., Chailakhjan R. K. & Lalykina K. S. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet 3, 393–403 (1970). - PubMed
-
- Bianco P. “Mesenchymal” stem cells. Annu Rev Cell Dev Biol 30, 677–704 (2014). - PubMed
-
- Caplan A. I. Mesenchymal stem cells. J Orthop Res 9, 641–650 (1991). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

