Temporal order of RNase IIIb and loss-of-function mutations during development determines phenotype in pleuropulmonary blastoma / DICER1 syndrome: a unique variant of the two-hit tumor suppression model
- PMID: 26925222
- PMCID: PMC4712775
- DOI: 10.12688/f1000research.6746.2
Temporal order of RNase IIIb and loss-of-function mutations during development determines phenotype in pleuropulmonary blastoma / DICER1 syndrome: a unique variant of the two-hit tumor suppression model
Abstract
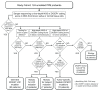
Pleuropulmonary blastoma (PPB) is the most frequent pediatric lung tumor and often the first indication of a pleiotropic cancer predisposition, DICER1 syndrome, comprising a range of other individually rare, benign and malignant tumors of childhood and early adulthood. The genetics of DICER1-associated tumorigenesis are unusual in that tumors typically bear neomorphic missense mutations at one of five specific "hotspot" codons within the RNase IIIb domain of DICER 1, combined with complete loss of function (LOF) in the other allele. We analyzed a cohort of 124 PPB children for predisposing DICER1 mutations and sought correlations with clinical phenotypes. Over 70% have inherited or de novo germline LOF mutations, most of which truncate the DICER1 open reading frame. We identified a minority of patients who have no germline mutation, but are instead mosaic for predisposing DICER1 mutations. Mosaicism for RNase IIIb domain hotspot mutations defines a special category of DICER1 syndrome patients, clinically distinguished from those with germline or mosaic LOF mutations by earlier onsets and numerous discrete foci of neoplastic disease involving multiple syndromic organ sites. A final category of PBB patients lack predisposing germline or mosaic mutations and have sporadic (rather than syndromic) disease limited to a single PPB tumor bearing tumor-specific RNase IIIb and LOF mutations. We propose that acquisition of a neomorphic RNase IIIb domain mutation is the rate limiting event in DICER1-associated tumorigenesis, and that distinct clinical phenotypes associated with mutational categories reflect the temporal order in which LOF and RNase IIIb domain mutations are acquired during development.
Keywords: DICER1 truncation; Mosaicism; PPB; Paediatric cancer; Pleuropulmonary blastoma; RNAse IIIb.
Conflict of interest statement
No competing interests were disclosed.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

