Inactivation of Anopheles gambiae Glutathione Transferase ε2 by Epiphyllocoumarin
- PMID: 26925266
- PMCID: PMC4746303
- DOI: 10.1155/2016/2516092
Inactivation of Anopheles gambiae Glutathione Transferase ε2 by Epiphyllocoumarin
Abstract
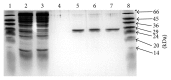
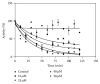
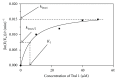
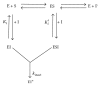
Glutathione transferases (GSTs) are part of a major family of detoxifying enzymes that can catalyze the reductive dehydrochlorination of dichlorodiphenyltrichloroethane (DDT). The delta and epsilon classes of insect GSTs have been implicated in conferring resistance to this insecticide. In this study, the inactivation of Anopheles gambiae GSTε2 by epiphyllocoumarin (Tral 1) was investigated. Recombinant AgGSTε2 was expressed in Escherichia coli cells containing a pET3a-AGSTε2 plasmid and purified by affinity chromatography. Tral 1 was shown to inactivate GSTε2 both in a time-dependent manner and in a concentration-dependent manner. The half-life of GSTε2 in the presence of 25 μM ethacrynic acid (ETA) was 22 minutes and with Tral 1 was 30 minutes, indicating that Tral 1 was not as efficient as ETA as an inactivator. The inactivation parameters k inact and K I were found to be 0.020 ± 0.001 min(-1) and 7.5 ± 2.1 μM, respectively, after 90 minutes of incubation. Inactivation of GSTε2 by Tral 1 implies that Tral 1 covalently binds to this enzyme in vitro and would be expected to exhibit time-dependent effects on the enzyme in vivo. Tral 1, therefore, would produce irreversible effects when used together with dichlorodiphenyltrichloroethane (DDT) in malaria control programmes where resistance is mediated by GSTs.
Figures


References
-
- Chen L., Hall P. R., Zhou X. E., Ranson H., Hemingway J., Meehan E. J. Structure of an insect δ-class glutathione S-transferase from a DDT-resistant strain of the malaria vector Anopheles gambiae . Acta Crystallographica—Section D: Biological Crystallography. 2003;59(12):2211–2217. doi: 10.1107/s0907444903018493. - DOI - PubMed
-
- Vermeulen N. P. E., Mulder G. J., Nieuwenhyse H., Peters W. H. M., Van Bladeren P. J. Glutathione S-Transferases: Structure, Function and Clinical Implications. London, UK: Taylor & Francis; 1996.
-
- Ding Y., Hawkes N., Meredith J., Eggleston P., Hemingway J., Ranson H. Characterization of the promoters of Epsilon glutathione transferases in the mosquito Anopheles gambiae and their response to oxidative stress. Biochemical Journal. 2005;387(3):879–888. doi: 10.1042/bj20041850. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

