Disruption of Glut1 in Hematopoietic Stem Cells Prevents Myelopoiesis and Enhanced Glucose Flux in Atheromatous Plaques of ApoE(-/-) Mice
- PMID: 26926469
- PMCID: PMC4824305
- DOI: 10.1161/CIRCRESAHA.115.307599
Disruption of Glut1 in Hematopoietic Stem Cells Prevents Myelopoiesis and Enhanced Glucose Flux in Atheromatous Plaques of ApoE(-/-) Mice
Abstract
Rationale: Inflamed atherosclerotic plaques can be visualized by noninvasive positron emission and computed tomographic imaging with (18)F-fluorodeoxyglucose, a glucose analog, but the underlying mechanisms are poorly understood.
Objective: Here, we directly investigated the role of Glut1-mediated glucose uptake in apolipoprotein E-deficient (ApoE(-/-)) mouse model of atherosclerosis.
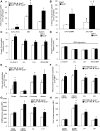
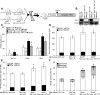
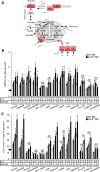
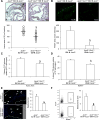
Methods and results: We first showed that the enhanced glycolytic flux in atheromatous plaques of ApoE(-/-) mice was associated with the enhanced metabolic activity of hematopoietic stem and multipotential progenitor cells and higher Glut1 expression in these cells. Mechanistically, the regulation of Glut1 in ApoE(-/-) hematopoietic stem and multipotential progenitor cells was not because of alterations in hypoxia-inducible factor 1α signaling or the oxygenation status of the bone marrow but was the consequence of the activation of the common β subunit of the granulocyte-macrophage colony-stimulating factor/interleukin-3 receptor driving glycolytic substrate utilization by mitochondria. By transplanting bone marrow from WT, Glut1(+/-), ApoE(-/-), and ApoE(-/-)Glut1(+/-) mice into hypercholesterolemic ApoE-deficient mice, we found that Glut1 deficiency reversed ApoE(-/-) hematopoietic stem and multipotential progenitor cell proliferation and expansion, which prevented the myelopoiesis and accelerated atherosclerosis of ApoE(-/-) mice transplanted with ApoE(-/-) bone marrow and resulted in reduced glucose uptake in the spleen and aortic arch of these mice.
Conclusions: We identified that Glut1 connects the enhanced glucose uptake in atheromatous plaques of ApoE(-/-) mice with their myelopoiesis through regulation of hematopoietic stem and multipotential progenitor cell maintenance and myelomonocytic fate and suggests Glut1 as potential drug target for atherosclerosis.
Keywords: atherosclerosis; bone marrow; cholesterol; glucose transporter type 1; glycolysis; myeloid cells.
© 2016 American Heart Association, Inc.
Conflict of interest statement
The authors have declared that no conflict of interest exists.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

