Multistrand Structure Prediction of Nucleic Acid Assemblies and Design of RNA Switches
- PMID: 26926528
- PMCID: PMC6319913
- DOI: 10.1021/acs.nanolett.5b04651
Multistrand Structure Prediction of Nucleic Acid Assemblies and Design of RNA Switches
Abstract
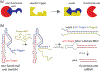
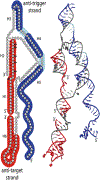
RNA is an attractive material for the creation of molecular logic gates that release programmed functionalities only in the presence of specific molecular interaction partners. Here we present HyperFold, a multistrand RNA/DNA structure prediction approach for predicting nucleic acid complexes that can contain pseudoknots. We show that HyperFold also performs competitively compared to other published folding algorithms. We performed a large variety of RNA/DNA hybrid reassociation experiments for different concentrations, DNA toehold lengths, and G+C content and find that the observed tendencies for reassociation correspond well to computational predictions. Importantly, we apply this method to the design and experimental verification of a two-stranded RNA molecular switch that upon binding to a single-stranded RNA toehold disease-marker trigger mRNA changes its conformation releasing an shRNA-like Dicer substrate structure. To demonstrate the concept, connective tissue growth factor (CTGF) mRNA and enhanced green fluorescent protein (eGFP) mRNA were chosen as trigger and target sequences, respectively. In vitro experiments confirm the formation of an RNA switch and demonstrate that the functional unit is being released when the trigger RNA interacts with the switch toehold. The designed RNA switch is shown to be functional in MDA-MB-231 breast cancer cells. Several other switches were also designed and tested. We conclude that this approach has considerable potential because, in principle, it allows the release of an siRNA designed against a gene that differs from the gene that is utilized as a biomarker for a disease state.
Keywords: Dicer; RNA interference; RNA switch; RNA/DNA hybrid; secondary structure.
Conflict of interest statement
Notes
The authors declare no competing financial interest.
Figures

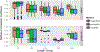


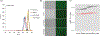
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

