Cyclic AMP Affects Oocyte Maturation and Embryo Development in Prepubertal and Adult Cattle
- PMID: 26926596
- PMCID: PMC4771806
- DOI: 10.1371/journal.pone.0150264
Cyclic AMP Affects Oocyte Maturation and Embryo Development in Prepubertal and Adult Cattle
Abstract
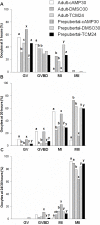
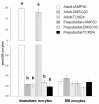
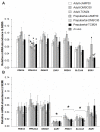
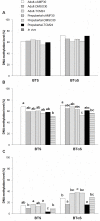
High cAMP levels during in vitro maturation (IVM) have been related to improved blastocyst yields. Here, we employed the cAMP/cGMP modulators, forskolin, IBMX, and cilostamide, during IVM to unravel the role of high cAMP in early embryonic development produced from prepubertal and adult bovine oocytes. Oocytes were collected via transvaginal aspiration and randomly assigned to three experimental groups: TCM24 (24 h IVM/control), cAMP30 (2 h pre-IVM (forskolin-IBMX), 30 h IVM-cilostamide), and DMSO30 (Dimethyl Sulfoxide/vehicle control). After IVM, oocytes were fertilized in vitro and zygotes were cultured in vitro to blastocysts. Meiotic progression, cAMP levels, mRNA abundance of selected genes and DNA methylation were evaluated in oocytes. Blastocysts were used for gene expression or DNA methylation analyses. Blastocysts from the cAMP30 groups were transferred to recipients. The cAMP elevation delayed meiotic progression, but developmental rates were not increased. In immature oocytes, mRNA abundance of PRKACA was higher for cAMP30 protocol and no differences were found for PDE3A, SMAD2, ZAR1, PRDX1 and SLC2A8. EGR1 gene was up-regulated in prepubertal cAMP30 immature oocytes and down-regulated in blastocysts from all in vitro treatments. A similar gene expression profile was observed for DNMT3b, BCL2L1, PRDX1 and SLC2A8 in blastocysts. Satellite DNA methylation profiles were different between prepubertal and adult oocytes and blastocysts derived from the TCM24 and DMSO30 groups. Blastocysts obtained from prepubertal and adult oocytes in the cAMP30 treatment displayed normal methylation profiles and produced offspring. These data indicate that cAMP regulates IVM in prepubertal and adult oocytes in a similar manner, with impact on the establishment of epigenetic marks and acquisition of full developmental competency.
Conflict of interest statement
Figures

References
-
- Shuhaibar LC, Egbert JR, Norris RP, Lampe PD, Nikolaev VO, Thunemann M, et al. Intercellular signaling via cyclic GMP diffusion through gap junctions restarts meiosis in mouse ovarian follicles. Proc Natl Acad Sci U S A. 2015;112(17):5527–32. Epub 2015/03/17. 10.1073/pnas.1423598112 ; PubMed Central PMCID: PMCPmc4418852. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

