RiMINI - the influence of rifaximin on minimal hepatic encephalopathy (MHE) and on the intestinal microbiome in patients with liver cirrhosis: study protocol for a randomized controlled trial
- PMID: 26926775
- PMCID: PMC4770677
- DOI: 10.1186/s13063-016-1205-8
RiMINI - the influence of rifaximin on minimal hepatic encephalopathy (MHE) and on the intestinal microbiome in patients with liver cirrhosis: study protocol for a randomized controlled trial
Abstract
Background: Hepatic encephalopathy (HE) is a clinically significant complication of liver cirrhosis impacting on the patients' quality of life. Minimal hepatic encephalopathy (MHE) is diagnosed by psychometric tests, found in up to 80 % of patients with liver cirrhosis and carries a high risk of progression to overt HE. Continuous therapy with rifaximin in combination with lactulose significantly reduces the risk of overt HE, recurrence of HE and HE-related hospitalizations in randomized, double-blind, placebo-controlled clinical trials. Rifaximin is approved for the therapy of overt HE in Germany. Treatment with lactulose has been shown to improve cognitive functions in patients with liver cirrhosis. Data from prospective clinical trials comparing the efficacy of rifaximin alone against a combination of rifaximin and lactulose in the treatment of MHE are scarce. Changes in the microbiome of the upper and lower gastrointestinal tract as a result of therapy with rifaximin have not yet been addressed in clinical studies.
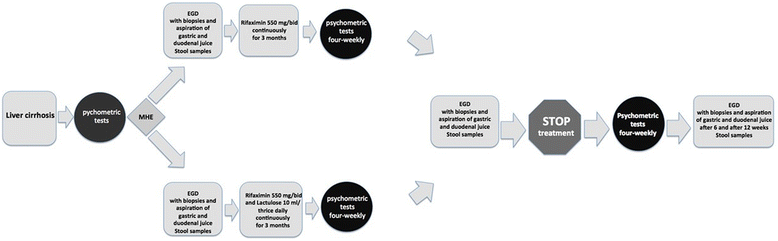
Methods and design: RiMINI is a monocentric exploratory pilot study on 60 patients with MHE as assessed by critical flicker frequency (CFF). Additionally, visual evoked potentials' (VEP) testing, electroencephalography (EEG) and psychometric testing (NCT-A) will be carried out. Patients will be randomized to treatment either with rifaximin alone (550 mg twice daily (bid) continuously for a period of 3 months) or with rifaximin (550 mg bid continuously) in combination with lactulose (30-60 ml daily) for 3 months. An esophagogastroduodenoscopy (EGD) will be performed at baseline, at the end of treatment and 6 and 12 weeks after the end of treatment to obtain gastric and duodenal biopsies and aspirates. The samples will be analyzed for their content of specific bacterial taxae by applying next generation sequencing (NGS) after rRNA isolation to identify the microbiome of the stomach and duodenum, and of the gut, in patients with liver cirrhosis and MHE before and after therapy.
Discussion: Differences of the effect of antibiotic therapy with rifaximin alone or in combination with lactulose on the clinical course of MHE are assessed.
Trial registration: The trial was registered as DRKS00006359 on March 17th 2015, with the universal trial number U1111-1163-9410 and with EudraCT2013-004414-18 .
References
-
- Ferenci P, Lockwood A, Mullen K, Tarter R, Weissenborn K, Blei AT. Hepatic encephalopathy – definition, nomenclature, diagnosis, and quantification: final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology. 2002;35(3):716–21. doi: 10.1053/jhep.2002.31250. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical