Nutritional Signaling Regulates Vitellogenin Synthesis and Egg Development through Juvenile Hormone in Nilaparvata lugens (Stål)
- PMID: 26927076
- PMCID: PMC4813133
- DOI: 10.3390/ijms17030269
Nutritional Signaling Regulates Vitellogenin Synthesis and Egg Development through Juvenile Hormone in Nilaparvata lugens (Stål)
Abstract
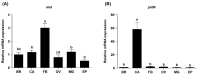
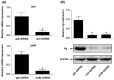
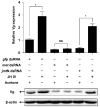
Insect female reproduction which comprises the synthesis of vitellogenein (Vg) in the fat body and its incorporation into developing oocytes, needs a large amount of energy and food resources. Our previous studies found that juvenile hormone (JH) regulates vitellogenesis in the brown planthopper, Nilaparvata lugens. Here, we report on the role of JH in nutrient-regulated Vg synthesis and egg development. We first cloned the genes coding for juvenile hormone acid methyltransferase (JHAMT) which is involved in JH biosynthesis and methoprene-tolerant (Met) for JH action. Amino acids (AAs) induced the expression of jmtN, while showing no effects on the expression of met using an artificial diet culture system. Reduction in JH biosynthesis or its action by RNA interference (RNAi)-mediated silencing of jmtN or met led to a severe inhibition of AAs-induced Vg synthesis and oocyte maturation, together with lower fecundity. Furthermore, exogenous application of JH III partially restored Vg expression levels in jmtN RNAi females. However, JH III application did not rescue Vg synthesis in these met RNAi insects. Our results show that AAs induce Vg synthesis in the fat body and egg development in concert with JH biosynthesis in Nilaparvata lugens (Stål), rather than through JH action.
Keywords: Juvenile hormone; Nilaparvata lugens; RNA inteference; nutritional signaling; vitellogenesis.
Figures



Similar articles
-
TOR Pathway-Mediated Juvenile Hormone Synthesis Regulates Nutrient-Dependent Female Reproduction in Nilaparvata lugens (Stål).Int J Mol Sci. 2016 Mar 28;17(4):438. doi: 10.3390/ijms17040438. Int J Mol Sci. 2016. PMID: 27043527 Free PMC article.
-
Molecular analysis of nutritional and hormonal regulation of female reproduction in the red flour beetle, Tribolium castaneum.Insect Biochem Mol Biol. 2011 May;41(5):294-305. doi: 10.1016/j.ibmb.2011.01.006. Epub 2011 Feb 1. Insect Biochem Mol Biol. 2011. PMID: 21288489 Free PMC article.
-
Molecular cloning, transcriptional regulation, and differential expression profiling of vitellogenin in two wing-morphs of the brown planthopper, Nilaparvata lugens Stål (Hemiptera: Delphacidae).Insect Mol Biol. 2010 Dec;19(6):787-98. doi: 10.1111/j.1365-2583.2010.01035.x. Insect Mol Biol. 2010. PMID: 20698901
-
Regulation of female reproduction in mites: a unifying model for the Acari.J Insect Physiol. 2009 Dec;55(12):1079-90. doi: 10.1016/j.jinsphys.2009.08.007. Epub 2009 Aug 28. J Insect Physiol. 2009. PMID: 19698719 Review.
-
Regulatory Mechanisms of Vitellogenesis in Insects.Front Cell Dev Biol. 2021 Jan 28;8:593613. doi: 10.3389/fcell.2020.593613. eCollection 2020. Front Cell Dev Biol. 2021. PMID: 33634094 Free PMC article. Review.
Cited by
-
Jinggangmycin-Induced UDP-Glycosyltransferase 1-2-Like Is a Positive Modulator of Fecundity and Population Growth in Nilaparvata lugens (Stål) (Hemiptera: Delphacidae).Front Physiol. 2019 Jun 21;10:747. doi: 10.3389/fphys.2019.00747. eCollection 2019. Front Physiol. 2019. PMID: 31293435 Free PMC article.
-
Combined analysis of the proteome and metabolome provides insight into microRNA-1174 function in Aedes aegypti mosquitoes.Parasit Vectors. 2023 Aug 9;16(1):271. doi: 10.1186/s13071-023-05859-1. Parasit Vectors. 2023. PMID: 37559132 Free PMC article.
-
Juvenile Hormone Synthesis Pathway Gene SfIPPI Regulates Sogatella furcifera Reproduction.Insects. 2022 Feb 6;13(2):174. doi: 10.3390/insects13020174. Insects. 2022. PMID: 35206747 Free PMC article.
-
A Genome-Wide Identification and Analysis of the Basic Helix-Loop-Helix Transcription Factors in Brown Planthopper, Nilaparvata lugens.Genes (Basel). 2016 Nov 18;7(11):100. doi: 10.3390/genes7110100. Genes (Basel). 2016. PMID: 27869716 Free PMC article.
-
Elevated temperatures diminish the effects of a highly resistant rice variety on the brown planthopper.Sci Rep. 2021 Jan 8;11(1):262. doi: 10.1038/s41598-020-80704-4. Sci Rep. 2021. PMID: 33420350 Free PMC article.
References
-
- Boldbaatar D., Battur B., Umemiya-Shirafuji R., Liao M., Tanaka T., Fujisaki K. GATA transcription, translation and regulation in Haemaphysalis. longicornis tick: Analysis of the cDNA and an essential role for vitellogenesis. Insect Biochem. Mol. Biol. 2010;40:49–57. doi: 10.1016/j.ibmb.2009.12.009. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

