Modulating Astrocyte Transition after Stroke to Promote Brain Rescue and Functional Recovery: Emerging Targets Include Rho Kinase
- PMID: 26927079
- PMCID: PMC4813152
- DOI: 10.3390/ijms17030288
Modulating Astrocyte Transition after Stroke to Promote Brain Rescue and Functional Recovery: Emerging Targets Include Rho Kinase
Abstract
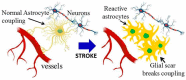
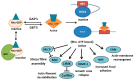
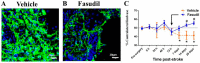
Stroke is a common and serious condition, with few therapies. Whilst previous focus has been directed towards biochemical events within neurons, none have successfully prevented the progression of injury that occurs in the acute phase. New targeted treatments that promote recovery after stroke might be a better strategy and are desperately needed for the majority of stroke survivors. Cells comprising the neurovascular unit, including blood vessels and astrocytes, present an alternative target for supporting brain rescue and recovery in the late phase of stroke, since alteration in the unit also occurs in regions outside of the lesion. One of the major changes in the unit involves extensive morphological transition of astrocytes resulting in altered energy metabolism, decreased glutamate reuptake and recycling, and retraction of astrocyte end feed from both blood vessels and neurons. Whilst globally inhibiting transitional change in astrocytes after stroke is reported to result in further damage and functional loss, we discuss the available evidence to suggest that the transitional activation of astrocytes after stroke can be modulated for improved outcomes. In particular, we review the role of Rho-kinase (ROCK) in reactive gliosis and show that inhibiting ROCK after stroke results in reduced scar formation and improved functional recovery.
Keywords: Rho-kinase inhibition; astrogliosis; connectivity; glial scar; neurovascular unit; regeneration.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

