Alternatively Activated Macrophages Boost Induced Regulatory T and Th17 Cell Responses during Immunotherapy for Colitis
- PMID: 26927797
- PMCID: PMC4851766
- DOI: 10.4049/jimmunol.1501956
Alternatively Activated Macrophages Boost Induced Regulatory T and Th17 Cell Responses during Immunotherapy for Colitis
Abstract
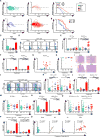
Induced regulatory T (iTreg) and Th17 cells promote mucosal homeostasis. We used a T cell transfer model of colitis to compare the capacity of iTreg and Th17 cells to develop in situ following the transfer of naive CD4(+)CD45RB(hi)T cells intoRag1(-/-)C57BL/6 or BALB/c mice, the prototypical Th1/M1- and Th2/M2-prone strains. We found that the frequency and number of Foxp3(+)iTreg cells and Th17 cells were significantly reduced in C57BL/6 mice compared with the BALB/c strain. C57BL/6 mice with colitis were also resistant to natural Treg cell immunotherapy. Pretreatment of C57BL/6Rag1(-/-)mice with IL-4 plus IL-13, or with M2a but not M1 macrophages, dramatically increased the generation of iTreg and Th17 cells. Importantly, M2a transfers, either as a pretreatment or in mice with established colitis, allowed successful immunotherapy with natural Treg cells. M2a macrophages also reduced the generation of pathogenic iTreg cells that lost Foxp3 expression, suggesting that they stabilize the expression of Foxp3. Thus, polarized M2a macrophages drive a directionally concordant expansion of the iTreg-Th17 cell axis and can be exploited as a therapeutic adjuvant in cell-transfer immunotherapy to re-establish mucosal tolerance.
Copyright © 2016 by The American Association of Immunologists, Inc.
Figures






References
-
- Haribhai D, Williams JB, Jia S, Nickerson D, Schmitt EG, Edwards B, Ziegelbauer J, Yassai M, Li SH, Relland LM, Wise PM, Chen A, Zheng YQ, Simpson PM, Gorski J, Salzman NH, Hessner MJ, Chatila TA, Williams CB. A requisite role for induced regulatory T cells in tolerance based on expanding antigen receptor diversity. Immunity. 2011;35:109–122. - PMC - PubMed
-
- Schmitt EG, Haribhai D, Williams JB, Aggarwal P, Jia S, Charbonnier LM, Yan K, Lorier R, Turner A, Ziegelbauer J, Georgiev P, Simpson P, Salzman NH, Hessner MJ, Broeckel U, Chatila TA, Williams CB. IL-10 Produced by Induced Regulatory T Cells (iTregs) Controls Colitis and Pathogenic Ex-iTregs during Immunotherapy. Journal of immunology. 2012;189:5638–5648. - PMC - PubMed
-
- Hsieh CS, Liang Y, Tyznik AJ, Self SG, Liggitt D, Rudensky AY. Recognition of the peripheral self by naturally arising CD25+ CD4+ T cell receptors. Immunity. 2004;21:267–277. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

