A Randomized Noninferiority Trial of Intravenous Iron Isomaltoside versus Oral Iron Sulfate in Patients with Nonmyeloid Malignancies and Anemia Receiving Chemotherapy: The PROFOUND Trial
- PMID: 26927900
- PMCID: PMC5071650
- DOI: 10.1002/phar.1729
A Randomized Noninferiority Trial of Intravenous Iron Isomaltoside versus Oral Iron Sulfate in Patients with Nonmyeloid Malignancies and Anemia Receiving Chemotherapy: The PROFOUND Trial
Abstract
Study objective: A safe alternative to erythropoiesis-stimulating agents to treat anemia is warranted in patients with cancer and anemia; thus the objective of this trial was to compare the efficacy and safety of intravenous (IV) iron isomaltoside with oral iron in patients with cancer and anemia by testing the noninferiority of IV versus oral iron.
Design: Phase III, prospective, open-label, comparative, randomized, noninferiority, multicenter trial.
Setting: Forty-seven hospitals or private cancer clinics in Asia, the United States, and Europe.
Patients: A total of 350 patients with cancer and anemia.
Intervention: Patients were randomized in a 2:1 ratio to either intravenous iron isomaltoside or oral iron sulfate. Patients in the iron isomaltoside group were then randomized into an infusion subgroup (single intravenous infusions of a maximum dose of 1000 mg over 15 min) or a bolus injection subgroup (bolus injections of 500 mg over 2 min).
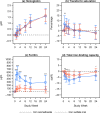
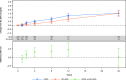
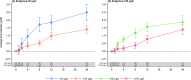
Measurements and main results: The primary efficacy outcome was change in hemoglobin concentration from baseline to week 4. Changes in other relevant hematology variables, effect on quality of life, and safety outcomes were also assessed. The primary efficacy outcome was tested for noninferiority, whereas the remaining outcomes were tested for superiority. Iron isomaltoside was noninferior to oral iron in change in hemoglobin concentration from baseline to week 4 (difference estimate 0.016, 95% confidence interval -0.26 to 0.29, p<0.001). A faster onset of the hemoglobin response was observed with infusion of iron isomaltoside (superiority test: p=0.03 at week 1), and a sustained effect on hemoglobin level was shown in both the iron isomaltoside and oral iron treatment groups until week 24. A significant mean decrease in fatigue score was observed from baseline to week 12 in the iron isomaltoside group (p<0.001) but not in the oral iron group (p=0.057). A higher proportion of patients treated with oral iron experienced adverse drug reactions (18.8% vs 6.6%, p<0.001) and discontinued the trial due to intolerance (8.0% vs 0.9%, p=0.001). Transient hypophosphatemia (phosphate level less than 2 mg/dl) was reported at similar low frequencies among the groups: 7.1% in the iron isomaltoside infusion subgroup versus 8.5% in the iron isomaltoside bolus injection subgroup versus 5.4% in the oral iron group.
Conclusion: This trial demonstrated comparable sustained increases in hemoglobin concentration over time with both iron isomaltoside and oral iron. Iron isomaltoside was better tolerated than oral iron, and fatigue was significantly decreased with iron isomaltoside. Low rates of clinically insignificant hypophosphatemia were reported in patients receiving both treatments.
Trial registration: ClinicalTrials.gov NCT01145638.
Keywords: anemia; cancer; iron isomaltoside; iron treatment.
© 2016 The Authors. Pharmacotherapy published by Wiley Periodicals, Inc. on behalf of Pharmacotherapy Publications, Inc.
Figures
Similar articles
-
Intravenous iron isomaltoside improves hemoglobin concentration and iron stores in female iron-deficient blood donors: a randomized double-blind placebo-controlled clinical trial.Transfusion. 2018 Apr;58(4):974-981. doi: 10.1111/trf.14521. Epub 2018 Feb 9. Transfusion. 2018. PMID: 29424441 Clinical Trial.
-
Effects of Iron Isomaltoside vs Ferric Carboxymaltose on Hypophosphatemia in Iron-Deficiency Anemia: Two Randomized Clinical Trials.JAMA. 2020 Feb 4;323(5):432-443. doi: 10.1001/jama.2019.22450. JAMA. 2020. PMID: 32016310 Free PMC article. Clinical Trial.
-
Iron isomaltoside 1000: a new intravenous iron for treating iron deficiency in chronic kidney disease.J Nephrol. 2011 Sep-Oct;24(5):589-96. doi: 10.5301/JN.2011.6248. J Nephrol. 2011. PMID: 21240875 Clinical Trial.
-
A systematic literature review and indirect comparison of iron isomaltoside and ferric carboxymaltose in iron deficiency anemia after failure or intolerance of oral iron treatment.Expert Rev Hematol. 2019 Feb;12(2):129-136. doi: 10.1080/17474086.2019.1575202. Epub 2019 Feb 19. Expert Rev Hematol. 2019. PMID: 30689458
-
Use of intravenous iron supplementation in chronic kidney disease: an update.Iran J Kidney Dis. 2013 Jan;7(1):9-22. Iran J Kidney Dis. 2013. PMID: 23314137 Review.
Cited by
-
Safety and efficacy of iron isomaltoside 1000/ferric derisomaltose versus iron sucrose in patients with chronic kidney disease: the FERWON-NEPHRO randomized, open-label, comparative trial.Nephrol Dial Transplant. 2021 Jan 1;36(1):111-120. doi: 10.1093/ndt/gfaa011. Nephrol Dial Transplant. 2021. PMID: 32049331 Free PMC article. Clinical Trial.
-
Real-world evaluation of an intravenous iron service for the treatment of iron deficiency with or without anemia.Sci Rep. 2025 Apr 9;15(1):12093. doi: 10.1038/s41598-025-85880-9. Sci Rep. 2025. PMID: 40204729 Free PMC article.
-
Characterization of serum phosphate levels over time with intravenous ferric carboxymaltose versus placebo as treatment for heart failure with reduced ejection fraction and iron deficiency: An exploratory prospective substudy from HEART-FID.Eur J Heart Fail. 2025 May;27(5):872-880. doi: 10.1002/ejhf.3348. Epub 2024 Jun 19. Eur J Heart Fail. 2025. PMID: 38896006 Free PMC article. Clinical Trial.
-
Flipside of the Coin: Iron Deficiency and Colorectal Cancer.Front Immunol. 2021 Mar 11;12:635899. doi: 10.3389/fimmu.2021.635899. eCollection 2021. Front Immunol. 2021. PMID: 33777027 Free PMC article. Review.
-
Intravenous Iron Supplementation for the Treatment of Chemotherapy-Induced Anemia: A Systematic Review and Meta-Analysis of Randomized Controlled Trials.J Clin Med. 2022 Jul 18;11(14):4156. doi: 10.3390/jcm11144156. J Clin Med. 2022. PMID: 35887920 Free PMC article. Review.
References
-
- Groopman JE, Itri LM. Chemotherapy‐induced anemia in adults: Incidence and treatment. J Natl Cancer Inst 1999;91:1616–34. - PubMed
-
- Ludwig H, Van BS, Barrett‐Lee P, et al. The European Cancer Anaemia Survey (ECAS): a large, multinational, prospective survey defining the prevalence, incidence, and treatment of anaemia in cancer patients. Eur J Cancer 2004;40:2293–306. - PubMed
-
- Henry DH. The role of intravenous iron in cancer‐related anemia. Oncology (Williston Park) 2006;20(8 Suppl 6):21–4. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

