Integrase inhibitors in late pregnancy and rapid HIV viral load reduction
- PMID: 26928154
- PMCID: PMC4995881
- DOI: 10.1016/j.ajog.2015.12.052
Integrase inhibitors in late pregnancy and rapid HIV viral load reduction
Abstract
Background: Minimizing time to HIV viral suppression is critical in pregnancy. Integrase strand transfer inhibitors (INSTIs), like raltegravir, are known to rapidly suppress plasma HIV RNA in nonpregnant adults. There are limited data in pregnant women.
Objective: We describe time to clinically relevant reduction in HIV RNA in pregnant women using INSTI-containing and non-INSTI-containing antiretroviral therapy (ART) options.
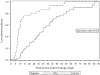
Study design: We conducted a retrospective cohort study of pregnant HIV-infected women in the United States from 2009 through 2015. We included women who initiated ART, intensified their regimen, or switched to a new regimen due to detectable viremia (HIV RNA >40 copies/mL) at ≥20 weeks gestation. Among women with a baseline HIV RNA permitting 1-log reduction, we estimated time to 1-log RNA reduction using the Kaplan-Meier estimator comparing women starting/adding an INSTI in their regimen vs other ART. To compare groups with similar follow-up time, we also conducted a subgroup analysis limited to women with ≤14 days between baseline and follow-up RNA data.
Results: This study describes 101 HIV-infected pregnant women from 11 US clinics. In all, 75% (76/101) of women were not taking ART at baseline; 24 were taking non-INSTI containing ART, and 1 received zidovudine monotherapy. In all, 39% (39/101) of women started an INSTI-containing regimen or added an INSTI to their ART regimen. Among 90 women with a baseline HIV RNA permitting 1-log reduction, the median time to 1-log RNA reduction was 8 days (interquartile range [IQR], 7-14) in the INSTI group vs 35 days (IQR, 20-53) in the non-INSTI ART group (P < .01). In a subgroup of 39 women with first and last RNA measurements ≤14 days apart, median time to 1-log reduction was 7 days (IQR, 6-10) in the INSTI group vs 11 days (IQR, 10-14) in the non-INSTI group (P < .01).
Conclusion: ART that includes INSTIs appears to induce more rapid viral suppression than other ART regimens in pregnancy. Inclusion of an INSTI may play a role in optimal reduction of HIV RNA for HIV-infected pregnant women presenting late to care or failing initial therapy. Larger studies are urgently needed to assess the safety and effectiveness of this approach.
Keywords: HIV; integrase inhibitors; pregnancy.
Copyright © 2016 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors report no relevant financial conflicts of interest.
Figures

Similar articles
-
Durability of rilpivirine-based versus integrase inhibitor-based regimens in a large cohort of naïve HIV-infected patients starting antiretroviral therapy.Int J Antimicrob Agents. 2021 Oct;58(4):106406. doi: 10.1016/j.ijantimicag.2021.106406. Epub 2021 Jul 19. Int J Antimicrob Agents. 2021. PMID: 34293454
-
Weight gain among treatment-naïve persons with HIV starting integrase inhibitors compared to non-nucleoside reverse transcriptase inhibitors or protease inhibitors in a large observational cohort in the United States and Canada.J Int AIDS Soc. 2020 Apr;23(4):e25484. doi: 10.1002/jia2.25484. J Int AIDS Soc. 2020. PMID: 32294337 Free PMC article.
-
Time spent with residual viraemia after virological suppression below 50 HIV-RNA copies/mL according to type of first-line antiretroviral regimen.Int J Antimicrob Agents. 2018 Oct;52(4):492-499. doi: 10.1016/j.ijantimicag.2018.07.001. Epub 2018 Sep 13. Int J Antimicrob Agents. 2018. PMID: 30009958
-
Prevalence of Emergent Dolutegravir Resistance Mutations in People Living with HIV: A Rapid Scoping Review.Viruses. 2024 Mar 4;16(3):399. doi: 10.3390/v16030399. Viruses. 2024. PMID: 38543764 Free PMC article.
-
Dolutegravir, a second-generation integrase inhibitor for the treatment of HIV-1 infection.Ann Pharmacother. 2014 Mar;48(3):395-403. doi: 10.1177/1060028013513558. Epub 2013 Nov 19. Ann Pharmacother. 2014. PMID: 24259658 Review.
Cited by
-
Raltegravir-intensified initial antiretroviral therapy in advanced HIV disease in Africa: A randomised controlled trial.PLoS Med. 2018 Dec 4;15(12):e1002706. doi: 10.1371/journal.pmed.1002706. eCollection 2018 Dec. PLoS Med. 2018. PMID: 30513108 Free PMC article. Clinical Trial.
-
Antiretroviral Regimen and Pregnancy Outcomes of Women Living with HIV in a US Cohort.Infect Dis Clin Pract (Baltim Md). 2023 Nov;31(6):e1308. doi: 10.1097/IPC.0000000000001308. Epub 2023 Sep 25. Infect Dis Clin Pract (Baltim Md). 2023. PMID: 38213314 Free PMC article.
-
Accelerating progress towards the elimination of mother-to-child transmission of HIV: a narrative review.J Int AIDS Soc. 2020 Aug;23(8):e25571. doi: 10.1002/jia2.25571. J Int AIDS Soc. 2020. PMID: 32820609 Free PMC article. Review.
-
Cost-effectiveness of dolutegravir vs. efavirenz-based combined antiretroviral therapies in HIV-infected treatment-naive patients in a Nigerian treatment centre.Afr Health Sci. 2023 Mar;23(1):157-169. doi: 10.4314/ahs.v23i1.18. Afr Health Sci. 2023. PMID: 37545946 Free PMC article.
-
The Predictive Value of Lactate Dehydrogenase for Viral Suppression in Newly Diagnosed People Living With HIV on Antiretroviral Therapy: A Retrospective Cohort Study.Infect Drug Resist. 2025 Jan 30;18:601-611. doi: 10.2147/IDR.S488220. eCollection 2025. Infect Drug Resist. 2025. PMID: 39902274 Free PMC article.
References
-
- Panel of Treatment of HIV-Infected Pregnant Women and Prevention of Perinatal Transmission. Recommendations for use of antiretroviral drugs in pregnant HIV-1-infected women for maternal health and interventions to reduce perinatal HIV transmission in the United States. 2015 http://aidsinfo.nih.gov/contentfiles/lvguidelines/PerinatalGL.pdf. Accessed Aug 8, 2015. - PubMed
-
- Townsend CL, Cortina-Borja M, Peckem CS, de Ruiter A, Lyall H, Tookey PA. Low rates of mother-to-child transmission of HIV following effective pregnancy intervention in the United Kingdom and Ireland, 2000–2006. AIDS. 2008;22:973–981. - PubMed
-
- Neisham S, Taylor A, Lampe MA, et al. A framework for elimination of perinatal transmission of HIV in the United States. Pediatrics. 2012;130:738–744. - PubMed
-
- Connor EM, Sperling RS, Gelber R, et al. Reduction of maternal-infact transmision of human immunodeficiency virus type 1 with zidovudine treatment. Pediatri AIDS Clinical Trials Group Protocol 076 Study Group. N Engl J Med. 1994;331:1173–1180. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

