Redox regulation of epidermal growth factor receptor signaling during the development of pulmonary hypertension
- PMID: 26928584
- PMCID: PMC5929487
- DOI: 10.1016/j.freeradbiomed.2016.02.029
Redox regulation of epidermal growth factor receptor signaling during the development of pulmonary hypertension
Abstract
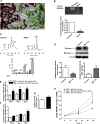
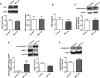
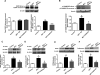
The development of pulmonary hypertension (PH) involves the uncontrolled proliferation of pulmonary smooth muscle cells via increased growth factor receptor signaling. However, the role of epidermal growth factor receptor (EGFR) signaling is controversial, as humans with advanced PH exhibit no changes in EGFR protein levels and purpose of the present study was to determine whether there are post-translational mechanisms that enhance EGFR signaling in PH. The EGFR inhibitor, gefinitib, significantly attenuated EGFR signaling and prevented the development of PH in monocrotaline (MCT)-exposed rats, confirming the contribution of EGFR activation in MCT induced PH. There was an early MCT-mediated increase in hydrogen peroxide, which correlated with the binding of the active metabolite of MCT, monocrotaline pyrrole, to catalase Cys377, disrupting its multimeric structure. This early oxidative stress was responsible for the oxidation of EGFR and the formation of sodium dodecyl sulfate (SDS) stable EGFR dimers through dityrosine cross-linking. These cross-linked dimers exhibited increased EGFR autophosphorylation and signaling. The activation of EGFR signaling did not correlate with pp60(src) dependent Y845 phosphorylation or EGFR ligand expression. Importantly, the analysis of patients with advanced PH revealed the same enhancement of EGFR autophosphorylation and covalent dimer formation in pulmonary arteries, while total EGFR protein levels were unchanged. As in the MCT exposed rat model, the activation of EGFR in human samples was independent of pp60(src) phosphorylation site and ligand expression. This study provides a novel molecular mechanism of oxidative stress stimulated covalent EGFR dimerization via tyrosine dimerization that contributes into development of PH.
Keywords: Catalase; EGFR; Oxidative stress; Proliferation; Pulmonary hypertension.
Copyright © 2016. Published by Elsevier Inc.
Figures





References
-
- Bowers R, Cool C, Murphy RC, Tuder RM, Hopken MW, Flores SC, Voelkel NF. Oxidative stress in severe pulmonary hypertension. Am. J. Respir. Crit. Care Med. 2004;169:764–769. - PubMed
-
- Perez-Vizcaino F, Cogolludo A, Moreno L. Reactive oxygen species signaling in pulmonary vascular smooth muscle. Respir. Physiol. Neurobiol. 2010;174:212–220. - PubMed
-
- Higgins P, Dawson J, Lees KR, McArthur K, Quinn TJ, Walters MR. Xanthine oxidase inhibition for the treatment of cardiovascular disease: a systematic review and meta-analysis. Cardiovasc. Ther. 2012;30:217–226. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

