Analysis of Mitochondrial Respiratory Chain Supercomplexes Using Blue Native Polyacrylamide Gel Electrophoresis (BN-PAGE)
- PMID: 26928661
- PMCID: PMC4823378
- DOI: 10.1002/9780470942390.mo150182
Analysis of Mitochondrial Respiratory Chain Supercomplexes Using Blue Native Polyacrylamide Gel Electrophoresis (BN-PAGE)
Abstract
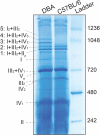
Mitochondria are cellular organelles that harvest energy in the form of ATP through a process termed oxidative phosphorylation (OXPHOS), which occurs via the protein complexes of the electron transport chain (ETC). In recent years it has become unequivocally clear that mitochondrial complexes of the ETC are not static entities in the inner mitochondrial membrane. These complexes are dynamic and in mammals they aggregate in different stoichiometric combinations to form supercomplexes (SCs) or respirasomes. It has been proposed that the net respiration is more efficient via SCs than via isolated complexes. However, it still needs to be determined whether the activity of a particular SC is associated with a disease etiology. Here we describe a simplified method to visualize and assess in-gel activity of SCs and the individual complexes with good resolution using blue native polyacrylamide gel electrophoresis (BN-PAGE).
Keywords: Cox7a2l; SCAFI; in-gel activity; mitochondria; oxidative phosphorylation; supercomplex.
Copyright © 2016 John Wiley & Sons, Inc.
Figures




References
-
- Acin-Perez R, Fernandez-Silva P, Peleato ML, Perez-Martos A, Enriquez JA. Respiratory active mitochondrial supercomplexes. Molecular cell. 2008;32:529–539. - PubMed
-
- Adams S, Pacharinsak C. Mouse anesthesia and analgesia. Current protocols in mouse biology. 2015;5:51–63. - PubMed
-
- Budde SM, van den Heuvel LP, Janssen AJ, Smeets RJ, Buskens CA, DeMeirleir L, Van Coster R, Baethmann M, Voit T, Trijbels JM, Smeitink JA. Combined enzymatic complex I and III deficiency associated with mutations in the nuclear encoded NDUFS4 gene. Biochemical and biophysical research communications. 2000;275:63–68. - PubMed
-
- Chaban Y, Boekema EJ, Dudkina NV. Structures of mitochondrial oxidative phosphorylation supercomplexes and mechanisms for their stabilisation. Biochimica et biophysica acta. 2014;1837:418–426. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

