Novel approaches to the management of noneosinophilic asthma
- PMID: 26929306
- PMCID: PMC5933607
- DOI: 10.1177/1753465816632638
Novel approaches to the management of noneosinophilic asthma
Abstract
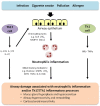
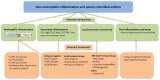
Noneosinophilic airway inflammation occurs in approximately 50% of patients with asthma. It is subdivided into neutrophilic or paucigranulocytic inflammation, although the proportion of each subtype is uncertain because of variable cut-off points used to define neutrophilia. This article reviews the evidence for noneosinophilic inflammation being a target for therapy in asthma and assesses clinical trials of licensed drugs, novel small molecules and biologics agents in noneosinophilic inflammation. Current symptoms, rate of exacerbations and decline in lung function are generally less in noneosinophilic asthma than eosinophilic asthma. Noneosinophilic inflammation is associated with corticosteroid insensitivity. Neutrophil activation in the airways and systemic inflammation is reported in neutrophilic asthma. Neutrophilia in asthma may be due to corticosteroids, associated chronic pulmonary infection, altered airway microbiome or delayed neutrophil apoptosis. The cause of poorly controlled noneosinophilic asthma may differ between patients and involve several mechanism including neutrophilic inflammation, T helper 2 (Th2)-low or other subtypes of airway inflammation or corticosteroid insensitivity as well as noninflammatory pathways such as airway hyperreactivity and remodelling. Smoking cessation in asthmatic smokers and removal from exposure to some occupational agents reduces neutrophilic inflammation. Preliminary studies of 'off-label' use of licensed drugs suggest that macrolides show efficacy in nonsmokers with noneosinophilic severe asthma and statins, low-dose theophylline and peroxisome proliferator-activated receptor gamma (PPARγ) agonists may benefit asthmatic smokers with noneosinophilic inflammation. Novel small molecules targeting neutrophilic inflammation, such as chemokine (CXC) receptor 2 (CXCR2) antagonists reduce neutrophils, but do not improve clinical outcomes in studies to date. Inhaled phosphodiesterase (PDE)4 inhibitors, dual PDE3 and PDE4 inhibitors, p38MAPK (mitogen-activated protein kinase) inhibitors, tyrosine kinase inhibitors and PI (phosphoinositide) 3kinase inhibitors are under development and these compounds may be of benefit in noneosinophilic inflammation. The results of clinical trials of biological agents targeting mediators associated with noneosinophilic inflammation, such as interleukin (IL)-17 and tumor necrosis factor (TNF)-α are disappointing. Greater understanding of the mechanisms of noneosinophilic inflammation in asthma should lead to improved therapies.
Keywords: airway inflammation; asthma; biological agents; biomarkers; cigarette smoking; corticosteroid insensitivity; eosinophils; neutrophils; small molecules.
© The Author(s), 2016.
Conflict of interest statement
Figures
References
-
- Al-Samri M., Benedetti A., Préfontaine D., Olivenstein R., Lemière C., Nair P., et al. (2010) Variability of sputum inflammatory cells in asthmatic patients receiving corticosteroid therapy: a prospective study using multiple samples. J Allergy Clin Immunol 125: 1161–1163. - PubMed
-
- Arron J., Choy D., Laviolette M., Kelsen S., Hatab A., Leigh R., et al. (2014) Disconnect between sputum neutrophils and other measures of airway inflammation in asthma. Eur Respir J 43: 627–629. - PubMed
-
- Arron J., Scheerens H., Matthews J., David R. (2013) Redefining approaches to asthma: developing targeted biologic therapies. Adv Pharmacol - Immunopharmacol 66: 1–49. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

