Ablation of sensory neurons in a genetic model of pancreatic ductal adenocarcinoma slows initiation and progression of cancer
- PMID: 26929329
- PMCID: PMC4801275
- DOI: 10.1073/pnas.1512603113
Ablation of sensory neurons in a genetic model of pancreatic ductal adenocarcinoma slows initiation and progression of cancer
Abstract
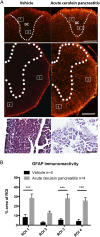
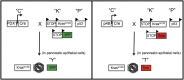
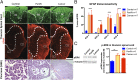
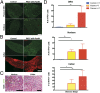
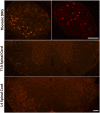
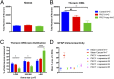
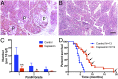
Pancreatic ductal adenocarcinoma (PDAC) is characterized by an exuberant inflammatory desmoplastic response. The PDAC microenvironment is complex, containing both pro- and antitumorigenic elements, and remains to be fully characterized. Here, we show that sensory neurons, an under-studied cohort of the pancreas tumor stroma, play a significant role in the initiation and progression of the early stages of PDAC. Using a well-established autochthonous model of PDAC (PKC), we show that inflammation and neuronal damage in the peripheral and central nervous system (CNS) occurs as early as the pancreatic intraepithelial neoplasia (PanIN) 2 stage. Also at the PanIN2 stage, pancreas acinar-derived cells frequently invade along sensory neurons into the spinal cord and migrate caudally to the lower thoracic and upper lumbar regions. Sensory neuron ablation by neonatal capsaicin injection prevented perineural invasion (PNI), astrocyte activation, and neuronal damage, suggesting that sensory neurons convey inflammatory signals from Kras-induced pancreatic neoplasia to the CNS. Neuron ablation in PKC mice also significantly delayed PanIN formation and ultimately prolonged survival compared with vehicle-treated controls (median survival, 7.8 vs. 4.5 mo; P = 0.001). These data establish a reciprocal signaling loop between the pancreas and nervous system, including the CNS, that supports inflammation associated with oncogenic Kras-induced neoplasia. Thus, pancreatic sensory neurons comprise an important stromal cell population that supports the initiation and progression of PDAC and may represent a potential target for prevention in high-risk populations.
Keywords: PanIN; inflammation; pancreatic ductal adenocarcinoma; sensory neuron; tumorigenesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Comment in
-
Pancreatic cancer: Sensory neuron ablation ameliorates PDAC.Nat Rev Gastroenterol Hepatol. 2016 May;13(5):250-1. doi: 10.1038/nrgastro.2016.48. Epub 2016 Mar 16. Nat Rev Gastroenterol Hepatol. 2016. PMID: 27006249 No abstract available.
References
-
- Carobi C. Capsaicin-sensitive vagal afferent neurons innervating the rat pancreas. Neurosci Lett. 1987;77(1):5–9. - PubMed
-
- Lindsay TH, et al. Pancreatic cancer pain and its correlation with changes in tumor vasculature, macrophage infiltration, neuronal innervation, body weight and disease progression. Pain. 2005;119(1-3):233–246. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

