Critical role for Epac1 in inflammatory pain controlled by GRK2-mediated phosphorylation of Epac1
- PMID: 26929333
- PMCID: PMC4801297
- DOI: 10.1073/pnas.1516036113
Critical role for Epac1 in inflammatory pain controlled by GRK2-mediated phosphorylation of Epac1
Abstract
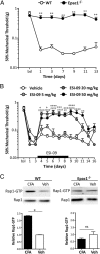
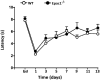
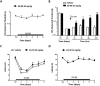
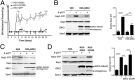
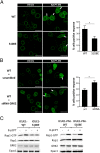
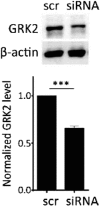
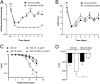
cAMP signaling plays a key role in regulating pain sensitivity. Here, we uncover a previously unidentified molecular mechanism in which direct phosphorylation of the exchange protein directly activated by cAMP 1 (EPAC1) by G protein kinase 2 (GRK2) suppresses Epac1-to-Rap1 signaling, thereby inhibiting persistent inflammatory pain. Epac1(-/-) mice are protected against inflammatory hyperalgesia in the complete Freund's adjuvant (CFA) model. Moreover, the Epac-specific inhibitor ESI-09 inhibits established CFA-induced mechanical hyperalgesia without affecting normal mechanical sensitivity. At the mechanistic level, CFA increased activity of the Epac target Rap1 in dorsal root ganglia of WT, but not of Epac1(-/-), mice. Using sensory neuron-specific overexpression of GRK2 or its kinase-dead mutant in vivo, we demonstrate that GRK2 inhibits CFA-induced hyperalgesia in a kinase activity-dependent manner. In vitro, GRK2 inhibits Epac1-to-Rap1 signaling by phosphorylation of Epac1 at Ser-108 in the Disheveled/Egl-10/pleckstrin domain. This phosphorylation event inhibits agonist-induced translocation of Epac1 to the plasma membrane, thereby reducing Rap1 activation. Finally, we show that GRK2 inhibits Epac1-mediated sensitization of the mechanosensor Piezo2 and that Piezo2 contributes to inflammatory mechanical hyperalgesia. Collectively, these findings identify a key role of Epac1 in chronic inflammatory pain and a molecular mechanism for controlling Epac1 activity and chronic pain through phosphorylation of Epac1 at Ser-108. Importantly, using the Epac inhibitor ESI-09, we validate Epac1 as a potential therapeutic target for chronic pain.
Keywords: Epac1; Epac1 translocation; GRK2; Piezo2; chronic pain.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- Simon LS. Relieving pain in America: A blueprint for transforming prevention, care, education, and research. J Pain Palliat Care Pharmacother. 2012;26(2):197–198.
-
- Wei F, et al. Genetic elimination of behavioral sensitization in mice lacking calmodulin-stimulated adenylyl cyclases. Neuron. 2002;36(4):713–726. - PubMed
-
- Eijkelkamp N, Singhmar P, Heijnen CJ, Kavelaars A. 2015. Sensory neuron cAMP signaling in chronic pain. Cyclic Nucleotide Signaling, Methods in Signal Transduction Series, ed Cheng X (CRC, Boca Raton, FL), pp 113–134.
-
- Taiwo YO, Levine JD. Further confirmation of the role of adenyl cyclase and of cAMP-dependent protein kinase in primary afferent hyperalgesia. Neuroscience. 1991;44(1):131–135. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

