Disruption of lipid homeostasis in the Gram-negative cell envelope activates a novel cell death pathway
- PMID: 26929379
- PMCID: PMC4801249
- DOI: 10.1073/pnas.1601375113
Disruption of lipid homeostasis in the Gram-negative cell envelope activates a novel cell death pathway
Abstract
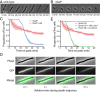
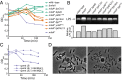
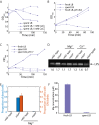
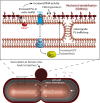
Gram-negative bacteria balance synthesis of the outer membrane (OM), cell wall, and cytoplasmic contents during growth via unknown mechanisms. Here, we show that a dominant mutation (designated mlaA*, maintenance of lipid asymmetry) that alters MlaA, a lipoprotein that removes phospholipids from the outer leaflet of the OM of Escherichia coli, increases OM permeability, lipopolysaccharide levels, drug sensitivity, and cell death in stationary phase. Surprisingly, single-cell imaging revealed that death occurs after protracted loss of OM material through vesiculation and blebbing at cell-division sites and compensatory shrinkage of the inner membrane, eventually resulting in rupture and slow leakage of cytoplasmic contents. The death of mlaA* cells was linked to fatty acid depletion and was not affected by membrane depolarization, suggesting that lipids flow from the inner membrane to the OM in an energy-independent manner. Suppressor analysis suggested that the dominant mlaA* mutation activates phospholipase A, resulting in increased levels of lipopolysaccharide and OM vesiculation that ultimately undermine the integrity of the cell envelope by depleting the inner membrane of phospholipids. This novel cell-death pathway suggests that balanced synthesis across both membranes is key to the mechanical integrity of the Gram-negative cell envelope.
Keywords: lipid transport; lipopolysaccharide; lysophospholipids; outer membrane; single-cell imaging.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

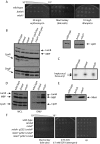
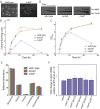
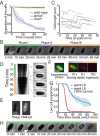
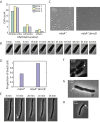

References
-
- Bos MP, Robert V, Tommassen J. Biogenesis of the Gram-negative bacterial outer membrane. Annu Rev Microbiol. 2007;61:191–214. - PubMed
-
- Vollmer W, Blanot D, De Pedro MA. Peptidoglycan structure and architecture. FEMS Microbiol Rev. 2008;32(2):149–167. - PubMed
-
- Navarro Llorens JM, Tormo A, Martínez-García E. Stationary phase in Gram-negative bacteria. FEMS Microbiol Rev. 2010;34(4):476–495. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

