The role of moxifloxacin in tuberculosis therapy
- PMID: 26929417
- PMCID: PMC9487659
- DOI: 10.1183/16000617.0085-2015
The role of moxifloxacin in tuberculosis therapy
Abstract
Tuberculosis (TB) remains a global threat with more than 9 million new infections. Treatment remains difficult and there has been no change in the duration of the standard regimen since the early 1980s. Moreover, many patients are unable to tolerate this treatment and discontinue therapy, increasing the risk of resistance. There is a growing tide of multidrug resistance and few effective antibiotics to tackle the problem. Since the turn of the millennium there has been a surge in interest in developing new therapies for TB and a number of new drugs have been developed. In this review the repurposing of moxifloxacin, an 8-methoxy-fluoroquinolone, for TB treatment is discussed. The evidence that underpins the development of this agent is reviewed. The results of the recently completed phase III trials are summarised and the reasons for the unexpected outcome are explored. Finally, the design of new trials that incorporate moxifloxacin, and that address both susceptible disease and multidrug resistance, is described.
Copyright ©ERS 2016.
Conflict of interest statement
Conflict of interest: Disclosures can be found alongside the online version of this article at
Figures
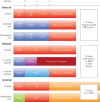
References
-
- Fox W. Whither short-course chemotherapy? Br J Dis Chest 1981; 75: 331–357. - PubMed
-
- Fox W, Ellard GA, Mitchison DA. Studies on the treatment of tuberculosis undertaken by the British Medical Research Council tuberculosis units, 1946–1986, with relevant subsequent publications. Int J Tuberc Lung Dis 1999; 3: S231–S279. - PubMed
-
- Zumla A, Nahid P, Cole S. Advances in the development of new tuberculosis drugs and treatment regimens. Nat Rev Drug Discov 2013; 12: 388–404. - PubMed
-
- Villemagne B, Crauste C, Flipo M, et al. . Tuberculosis: the drug development pipeline at a glance. Eur J Med Chem 2012; 51: 1–16. - PubMed
-
- Cohen J. Infectious disease. Approval of novel TB drug celebrated – with restraint. Science 2013; 339: 130. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
