Intra- and Interhemispheric Propagation of Electrophysiological Synchronous Activity and Its Modulation by Serotonin in the Cingulate Cortex of Juvenile Mice
- PMID: 26930051
- PMCID: PMC4773155
- DOI: 10.1371/journal.pone.0150092
Intra- and Interhemispheric Propagation of Electrophysiological Synchronous Activity and Its Modulation by Serotonin in the Cingulate Cortex of Juvenile Mice
Abstract
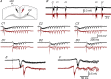
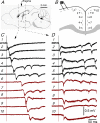
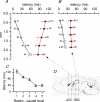
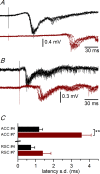
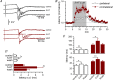
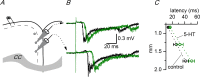
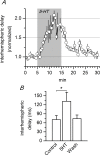
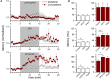
Disinhibition of the cortex (e.g., by GABA -receptor blockade) generates synchronous and oscillatory electrophysiological activity that propagates along the cortex. We have studied, in brain slices of the cingulate cortex of mice (postnatal age 14-20 days), the propagation along layer 2/3 as well as the interhemispheric propagation through the corpus callosum of synchronous discharges recorded extracellularly and evoked in the presence of 10 μM bicuculline by electrical stimulation of layer 1. The latency of the responses obtained at the same distance from the stimulus electrode was longer in anterior cingulate cortex (ACC: 39.53 ± 2.83 ms, n = 7) than in retrosplenial cortex slices (RSC: 21.99 ± 2.75 ms, n = 5; p<0.05), which is equivalent to a lower propagation velocity in the dorso-ventral direction in ACC than in RSC slices (43.0 mm/s vs 72.9 mm/s). We studied the modulation of this propagation by serotonin. Serotonin significantly increased the latency of the intracortical synchronous discharges (18.9% in the ipsilateral hemisphere and 40.2% in the contralateral hemisphere), and also increased the interhemispheric propagation time by 86.4%. These actions of serotonin were mimicked by the activation of either 5-HT1B or 5-HT2A receptors, but not by the activation of the 5-HT1A subtype. These findings provide further knowledge about the propagation of synchronic electrical activity in the cerebral cortex, including its modulation by serotonin, and suggest the presence of deep differences between the ACC and RSC in the structure of the local cortical microcircuits underlying the propagation of synchronous discharges.
Conflict of interest statement
Figures
Similar articles
-
Properties of the epileptiform activity in the cingulate cortex of a mouse model of LIS1 dysfunction.Brain Struct Funct. 2022 Jun;227(5):1599-1614. doi: 10.1007/s00429-022-02458-1. Epub 2022 Feb 1. Brain Struct Funct. 2022. PMID: 35103859 Free PMC article.
-
The antinociceptive effect of stimulating the retrosplenial cortex in the rat tail-flick test but not in the formalin test involves the rostral anterior cingulate cortex.Pharmacol Biochem Behav. 2015 Apr;131:112-8. doi: 10.1016/j.pbb.2015.02.006. Epub 2015 Feb 14. Pharmacol Biochem Behav. 2015. PMID: 25687372
-
Serotonergic modulation of neurotransmission in the rat basolateral amygdala.J Neurophysiol. 1999 Jul;82(1):69-85. doi: 10.1152/jn.1999.82.1.69. J Neurophysiol. 1999. PMID: 10400936
-
Spatiotemporal organization and thalamic modulation of seizures in the mouse medial thalamic-anterior cingulate slice.Epilepsia. 2011 Dec;52(12):2344-55. doi: 10.1111/j.1528-1167.2011.03312.x. Epub 2011 Nov 16. Epilepsia. 2011. PMID: 22092196
-
Antidepressant and anxiolytic effects of activating 5HT2A receptors in the anterior cingulate cortex and the theoretical mechanisms underlying them - A scoping review of available literature.Brain Res. 2025 Jan 1;1846:149226. doi: 10.1016/j.brainres.2024.149226. Epub 2024 Sep 7. Brain Res. 2025. PMID: 39251056
Cited by
-
Properties of the epileptiform activity in the cingulate cortex of a mouse model of LIS1 dysfunction.Brain Struct Funct. 2022 Jun;227(5):1599-1614. doi: 10.1007/s00429-022-02458-1. Epub 2022 Feb 1. Brain Struct Funct. 2022. PMID: 35103859 Free PMC article.
-
CB1-Dependent Long-Term Depression in Ventral Tegmental Area GABA Neurons: A Novel Target for Marijuana.J Neurosci. 2017 Nov 8;37(45):10943-10954. doi: 10.1523/JNEUROSCI.0190-17.2017. Epub 2017 Oct 16. J Neurosci. 2017. PMID: 29038246 Free PMC article.
-
Abnormalities in Cortical GABAergic Interneurons of the Primary Motor Cortex Caused by Lis1 (Pafah1b1) Mutation Produce a Non-drastic Functional Phenotype.Front Cell Dev Biol. 2022 Mar 2;10:769853. doi: 10.3389/fcell.2022.769853. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35309904 Free PMC article.
-
Optical Assay of the Functional Impact of Cuprizone-Induced Demyelination and Remyelination on Interhemispheric Neural Communication in the Anterior Cingulate Cortex via the Corpus Callosum.eNeuro. 2025 Jan 6;12(1):ENEURO.0511-24.2024. doi: 10.1523/ENEURO.0511-24.2024. Print 2025 Jan. eNeuro. 2025. PMID: 39746809 Free PMC article.
-
Synaptic Plasticity in the Pain-Related Cingulate and Insular Cortex.Biomedicines. 2022 Oct 28;10(11):2745. doi: 10.3390/biomedicines10112745. Biomedicines. 2022. PMID: 36359264 Free PMC article. Review.
References
-
- Vogt BA, Peters A. Form and distribution of neurons in rat cingulate cortex: areas 32, 24 and 29. J Comp Neurol 1981; 195:603–625. - PubMed
-
- Van Groen T, Wyss JM. Connections of the retrosplenial dysgranular cortex in the rat. J Comp Neurol 1992; 315:200–216. - PubMed
-
- Wyss JM, Van Groen T, Sripanidkulchai K. Dendritic bundling on layer I of granular retrosplenial cortex: intracellular labeling and selectivity of innervation. J Comp Neurol 1990; 295:33–42. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

