Distinct Contributions of Astrocytes and Pericytes to Neuroinflammation Identified in a 3D Human Blood-Brain Barrier on a Chip
- PMID: 26930059
- PMCID: PMC4773137
- DOI: 10.1371/journal.pone.0150360
Distinct Contributions of Astrocytes and Pericytes to Neuroinflammation Identified in a 3D Human Blood-Brain Barrier on a Chip
Abstract
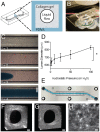
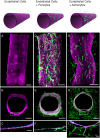
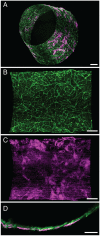
Neurovascular inflammation is a major contributor to many neurological disorders, but modeling these processes in vitro has proven to be difficult. Here, we microengineered a three-dimensional (3D) model of the human blood-brain barrier (BBB) within a microfluidic chip by creating a cylindrical collagen gel containing a central hollow lumen inside a microchannel, culturing primary human brain microvascular endothelial cells on the gel's inner surface, and flowing medium through the lumen. Studies were carried out with the engineered microvessel containing endothelium in the presence or absence of either primary human brain pericytes beneath the endothelium or primary human brain astrocytes within the surrounding collagen gel to explore the ability of this simplified model to identify distinct contributions of these supporting cells to the neuroinflammatory response. This human 3D BBB-on-a-chip exhibited barrier permeability similar to that observed in other in vitro BBB models created with non-human cells, and when stimulated with the inflammatory trigger, tumor necrosis factor-alpha (TNF-α), different secretion profiles for granulocyte colony-stimulating factor (G-CSF) and interleukin-6 (IL-6) were observed depending on the presence of astrocytes or pericytes. Importantly, the levels of these responses detected in the 3D BBB chip were significantly greater than when the same cells were co-cultured in static Transwell plates. Thus, as G-CSF and IL-6 have been reported to play important roles in neuroprotection and neuroactivation in vivo, this 3D BBB chip potentially offers a new method to study human neurovascular function and inflammation in vitro, and to identify physiological contributions of individual cell types.
Conflict of interest statement
Figures


References
-
- Minagar A, Alexander JS. Blood-brain barrier disruption in multiple sclerosis. Multiple sclerosis. 2003;9(6):540–9. - PubMed
-
- Wolburg H, Wolburg-Buchholz K, Kraus J, Rascher-Eggstein G, Liebner S, Hamm S, et al. Localization of claudin-3 in tight junctions of the blood-brain barrier is selectively lost during experimental autoimmune encephalomyelitis and human glioblastoma multiforme. Acta neuropathologica. 2003;105(6):586–92. - PubMed
-
- Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacological reviews. 2005;57(2):173–85. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

