Recombinant Measles AIK-C Vaccine Strain Expressing the prM-E Antigen of Japanese Encephalitis Virus
- PMID: 26930411
- PMCID: PMC4773129
- DOI: 10.1371/journal.pone.0150213
Recombinant Measles AIK-C Vaccine Strain Expressing the prM-E Antigen of Japanese Encephalitis Virus
Abstract
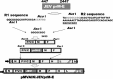
An inactivated Japanese encephalitis virus (JEV) vaccine, which induces neutralizing antibodies, has been used for many years in Japan. In the present study, the JEV prM-E protein gene was cloned, inserted at the P/M junction of measles AIK-C cDNA, and an infectious virus was recovered. The JEV E protein was expressed in B95a cells infected with the recombinant virus. Cotton rats were inoculated with recombinant virus. Measles PA antibodies were detected three weeks after immunization. Neutralizing antibodies against JEV developed one week after inoculation, and EIA antibodies were detected three weeks after immunization. The measles AIK-C-based recombinant virus simultaneously induced measles and JEV immune responses, and may be a candidate for infant vaccines. Therefore, the present strategy of recombinant viruses based on a measles vaccine vector would be applicable to the platform for vaccine development.
Conflict of interest statement
Figures




Similar articles
-
Vaccine platform recombinant measles virus.Virus Genes. 2017 Oct;53(5):733-740. doi: 10.1007/s11262-017-1486-3. Epub 2017 Jul 14. Virus Genes. 2017. PMID: 28710608 Free PMC article. Review.
-
Construction and immune efficacy of recombinant pseudorabies virus expressing PrM-E proteins of Japanese encephalitis virus genotype І.Virol J. 2015 Dec 10;12:214. doi: 10.1186/s12985-015-0449-3. Virol J. 2015. PMID: 26651827 Free PMC article.
-
Electroporation enhances protective immune response of a DNA vaccine against Japanese encephalitis in mice and pigs.Vaccine. 2016 Nov 11;34(47):5751-5757. doi: 10.1016/j.vaccine.2016.10.001. Epub 2016 Oct 13. Vaccine. 2016. PMID: 27743649
-
The immunogenicity of tetravalent dengue DNA vaccine in mice pre-exposed to Japanese encephalitis or Dengue virus antigens.Asian Pac J Allergy Immunol. 2015 Sep;33(3):182-8. doi: 10.12932/AP0508.33.3.2015. Asian Pac J Allergy Immunol. 2015. PMID: 26342114
-
Development of a recombinant vaccine against Japanese encephalitis.J Neurovirol. 2003 Aug;9(4):421-31. doi: 10.1080/13550280390218454. J Neurovirol. 2003. PMID: 12907387 Review.
Cited by
-
Vaccine platform recombinant measles virus.Virus Genes. 2017 Oct;53(5):733-740. doi: 10.1007/s11262-017-1486-3. Epub 2017 Jul 14. Virus Genes. 2017. PMID: 28710608 Free PMC article. Review.
-
A Measles Virus-Based Vaccine Candidate Mediates Protection against Zika Virus in an Allogeneic Mouse Pregnancy Model.J Virol. 2019 Jan 17;93(3):e01485-18. doi: 10.1128/JVI.01485-18. Print 2019 Feb 1. J Virol. 2019. PMID: 30429338 Free PMC article.
-
Chimeric Measles Virus (MV/RSV), Having Ectodomains of Respiratory Syncytial Virus (RSV) F and G Proteins Instead of Measles Envelope Proteins, Induced Protective Antibodies against RSV.Vaccines (Basel). 2021 Feb 16;9(2):156. doi: 10.3390/vaccines9020156. Vaccines (Basel). 2021. PMID: 33669275 Free PMC article.
-
Recombinant Measles AIK-C Vaccine Strain Expressing Influenza HA Protein.Vaccines (Basel). 2020 Mar 27;8(2):149. doi: 10.3390/vaccines8020149. Vaccines (Basel). 2020. PMID: 32230902 Free PMC article.
-
Development of Recombinant Measles Virus-Based Vaccines.Methods Mol Biol. 2017;1581:151-168. doi: 10.1007/978-1-4939-6869-5_9. Methods Mol Biol. 2017. PMID: 28374248 Free PMC article.
References
-
- WHO (2015) Japanese encephalitis vaccines: WHO position paper–February 2015. Wkly Epidemiol Rec 90: 69–88. - PubMed
-
- Halstead S, Jacobson J, Dubischar-Kastner K (2013) Japanese encephalitis vaccines In Plotkin S, Orenstein W, Offit P, editors. Vaccines 6 th edition, Saunders Elesvier; pp312–351.
-
- Konishi E, Shida M, Yamamoto S, Arai S, Tanaka-Taya K, Okabe N (2006) Natural infection with Japanese encephalitis virus among inhabitants of Japan: a nationwide survey of antibodies against nonstructural 1 protein. Vaccine 24: 3054–3056. - PubMed
-
- WHO Meeting report (2007) WHO informal consultation on the scientific basis for production of inactivated Japanese encephalitis vaccines for human use, Geneva, Switzerland, 1–2 June 2006. Vaccine 25: 5233–5243. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

