Increased choroidal mast cells and their degranulation in age-related macular degeneration
- PMID: 26931413
- PMCID: PMC4913697
- DOI: 10.1136/bjophthalmol-2015-308290
Increased choroidal mast cells and their degranulation in age-related macular degeneration
Abstract
Background/aims: Inflammation has been implicated in age-related macular degeneration (AMD). This study investigates the association of mast cells (MCs), a resident choroidal inflammatory cell, with pathological changes in AMD.
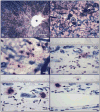
Methods: Human donor eyes included aged controls (n=10), clinically diagnosed with early AMD (n=8), geographic atrophy (GA, n=4) and exudative AMD (n=11). The choroids were excised and incubated for alkaline phosphatase (APase; blood vessels) and non-specific esterase activities (MCs). Degranulated (DG) and non-degranulated MCs in four areas of posterior choroid (nasal, non-macular, paramacular and submacular) were counted in flat mounts (4-6 fields/area). Choroids were subsequently embedded in JB-4 and sectioned for histological analyses.
Results: The number of MCs was significantly increased in all choroidal areas in early AMD (p=0.0006) and in paramacular area in exudative AMD (139.44±55.3 cells/mm(2); p=0.0091) and GA (199.08±82.0 cells/mm(2); p=0.0019) compared with the aged controls. DG MCs were also increased in paramacular (p=0.001) and submacular choroid (p=0.02) in all forms of AMD. Areas with the greatest numbers of DG MCs had loss of choriocapillaris (CC). Sections revealed that the MCs were widely distributed in Sattler's and Haller's layer in the choroidal stroma in aged controls, whereas MCs were frequently found in close proximity with CC in GA and exudative AMD and in choroidal neovascularisation (CNV).
Conclusion: Increased MC numbers and degranulation were observed in all AMD choroids. These results suggest that MC degranulation may contribute to the pathogenesis of AMD: death of CC and retinal pigment epithelial and CNV formation. The proteolytic enzymes released from MC granules may result in thinning of AMD choroid.
Keywords: Choroid; Inflammation; Macula; Neovascularisation; Pathology.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/
Figures



References
-
- Bhutto I, Lutty G. Understanding age-related macular degeneration (AMD): relationships between the photoreceptor/retinal pigment epithelium/Bruch's membrane/choriocapillaris complex. Molecular aspects of medicine. 2012;33(4):295–317. doi: 10.1016/j.mam.2012.04.005. published Online First: Epub Date. - DOI - PMC - PubMed
-
- Schramm EC, Clark SJ, Triebwasser MP, Raychaudhuri S, Seddon JM, Atkinson JP. Genetic variants in the complement system predisposing to age-related macular degeneration: a review. Molecular immunology. 2014;61(2):118–25. doi: 10.1016/j.molimm.2014.06.032. Online First: Epub Date. - DOI - PMC - PubMed
-
- Costa JJ, Weller PF, Galli SJ. The cells of the allergic response: mast cells, basophils, and eosinophils. JAMA. 1997;278(22):1815–22. - PubMed
-
- Galli SJ. Mast cells and basophils. Current opinion in hematology. 2000;7(1):32–9. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
