Mannose Receptor Is Required for Optimal Induction of Vaccine-Induced T-Helper Type 17 Cells and Resistance to Blastomyces dermatitidis Infection
- PMID: 26931447
- PMCID: PMC4857467
- DOI: 10.1093/infdis/jiw010
Mannose Receptor Is Required for Optimal Induction of Vaccine-Induced T-Helper Type 17 Cells and Resistance to Blastomyces dermatitidis Infection
Abstract
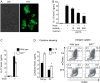
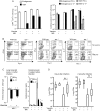
We investigated how innate sensing by the mannose receptor (MR) influences the development of antifungal immunity. We demonstrate that MR senses mannan on the surface of attenuated Blastomyces dermatitidis vaccine yeast and that MR(-/-) mice demonstrate impaired vaccine immunity against lethal experimental blastomycosis, compared with wild-type control mice. Using naive Blastomyces-specific transgenic CD4(+) T cells, we found that MR regulates differentiation of naive T cells into T-helper type 17 (Th17) effector cells, which are essential in vaccine immunity against systemic dimorphic fungi. Thus, MR regulates differentiation of Th17 cells and is required to induce vaccine immunity against lethal pulmonary blastomycosis.
Keywords: T-cell differentiation; fungi; mannose receptor; vaccine immunity.
© The Author 2016. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail journals.permissions@oup.com.
Figures
References
-
- Netea MG, Brown GD, Kullberg BJ, Gow NA. An integrated model of the recognition of Candida albicans by the innate immune system. Nat Rev Microbiol 2008; 6:67–78. - PubMed
-
- Levitz SM, Specht CA. The molecular basis for the immunogenicity of Cryptococcus neoformans mannoproteins. FEMS Yeast Res 2006; 6:513–24. - PubMed
-
- Saijo S, Ikeda S, Yamabe K et al. . Dectin-2 recognition of alpha-mannans and induction of Th17 cell differentiation is essential for host defense against Candida albicans. Immunity 2010; 32:681–91. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

