Frog Virus 3 dissemination in the brain of tadpoles, but not in adult Xenopus, involves blood brain barrier dysfunction
- PMID: 26931458
- PMCID: PMC4773881
- DOI: 10.1038/srep22508
Frog Virus 3 dissemination in the brain of tadpoles, but not in adult Xenopus, involves blood brain barrier dysfunction
Abstract
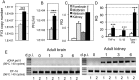
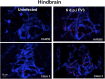
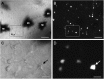
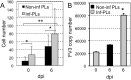
While increasing evidence points to a key role of monocytes in amphibian host defenses, monocytes are also thought to be important in the dissemination and persistent infection caused by ranavirus. However, little is known about the fate of infected macrophages or if ranavirus exploits immune privileged organs, such as the brain, in order to establish a reservoir. The amphibian Xenopus laevis and Frog Virus 3 (FV3) were established as an experimental platform for investigating in vivo whether ranavirus could disseminate to the brain. Our data show that the FV3 infection alters the BBB integrity, possibly mediated by an inflammatory response, which leads to viral dissemination into the central nervous system in X. laevis tadpole but not adult. Furthermore, our data suggest that the macrophages play a major role in viral dissemination by carrying the virus into the neural tissues.
Figures



Similar articles
-
Prominent amphibian (Xenopus laevis) tadpole type III interferon response to the frog virus 3 ranavirus.J Virol. 2015 May;89(9):5072-82. doi: 10.1128/JVI.00051-15. Epub 2015 Feb 25. J Virol. 2015. PMID: 25717104 Free PMC article.
-
The amphibian (Xenopus laevis) type I interferon response to frog virus 3: new insight into ranavirus pathogenicity.J Virol. 2014 May;88(10):5766-77. doi: 10.1128/JVI.00223-14. Epub 2014 Mar 12. J Virol. 2014. PMID: 24623410 Free PMC article.
-
Amphibian (Xenopus laevis) Tadpoles and Adult Frogs Differ in Their Antiviral Responses to Intestinal Frog Virus 3 Infections.Front Immunol. 2021 Aug 20;12:737403. doi: 10.3389/fimmu.2021.737403. eCollection 2021. Front Immunol. 2021. PMID: 34489981 Free PMC article.
-
Immune evasion strategies of ranaviruses and innate immune responses to these emerging pathogens.Viruses. 2012 Jul;4(7):1075-92. doi: 10.3390/v4071075. Epub 2012 Jun 28. Viruses. 2012. PMID: 22852041 Free PMC article. Review.
-
Amphibian macrophage development and antiviral defenses.Dev Comp Immunol. 2016 May;58:60-7. doi: 10.1016/j.dci.2015.12.008. Epub 2015 Dec 15. Dev Comp Immunol. 2016. PMID: 26705159 Free PMC article. Review.
Cited by
-
Xenopus-FV3 host-pathogen interactions and immune evasion.Virology. 2017 Nov;511:309-319. doi: 10.1016/j.virol.2017.06.005. Epub 2017 Jun 16. Virology. 2017. PMID: 28625407 Free PMC article.
-
Recombinant Ranaviruses for Studying Evolution of Host-Pathogen Interactions in Ectothermic Vertebrates.Viruses. 2016 Jul 6;8(7):187. doi: 10.3390/v8070187. Viruses. 2016. PMID: 27399758 Free PMC article. Review.
-
Form and Function of the Vertebrate and Invertebrate Blood-Brain Barriers.Int J Mol Sci. 2021 Nov 9;22(22):12111. doi: 10.3390/ijms222212111. Int J Mol Sci. 2021. PMID: 34829989 Free PMC article. Review.
-
Larval T Cells Are Functionally Distinct from Adult T Cells in Xenopus laevis.Immunohorizons. 2023 Oct 1;7(10):696-707. doi: 10.4049/immunohorizons.2300081. Immunohorizons. 2023. PMID: 37870488 Free PMC article.
-
Knockdown of NeuroD2 leads to seizure-like behavior, brain neuronal hyperactivity and a leaky blood-brain barrier in a Xenopus laevis tadpole model of DEE75.Genetics. 2024 Jul 8;227(3):iyae085. doi: 10.1093/genetics/iyae085. Genetics. 2024. PMID: 38788202 Free PMC article.
References
-
- Gray M. J., Miller D. L. & Hoverman J. T. Ecology and pathology of amphibian ranaviruses. Dis Aquat Organ 87, 243–266 (2009). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

