Cathepsin K induces platelet dysfunction and affects cell signaling in breast cancer - molecularly distinct behavior of cathepsin K in breast cancer
- PMID: 26931461
- PMCID: PMC4774035
- DOI: 10.1186/s12885-016-2203-7
Cathepsin K induces platelet dysfunction and affects cell signaling in breast cancer - molecularly distinct behavior of cathepsin K in breast cancer
Abstract
Background: Breast cancer comprises clinically and molecularly distinct tumor subgroups that differ in cell histology and biology and show divergent clinical phenotypes that impede phase III trials, such as those utilizing cathepsin K inhibitors. Here we correlate the epithelial-mesenchymal-like transition breast cancer cells and cathepsin K secretion with activation and aggregation of platelets. Cathepsin K is up-regulated in cancer cells that proteolyze extracellular matrix and contributes to invasiveness. Although proteolytically activated receptors (PARs) are activated by proteases, the direct interaction of cysteine cathepsins with PARs is poorly understood. In human platelets, PAR-1 and -4 are highly expressed, but PAR-3 shows low expression and unclear functions.
Methods: Platelet aggregation was monitored by measuring changes in turbidity. Platelets were immunoblotted with anti-phospho and total p38, Src-Tyr-416, FAK-Tyr-397, and TGFβ monoclonal antibody. Activation was measured in a flow cytometer and calcium mobilization in a confocal microscope. Mammary epithelial cells were prepared from the primary breast cancer samples of 15 women with Luminal-B subtype to produce primary cells.
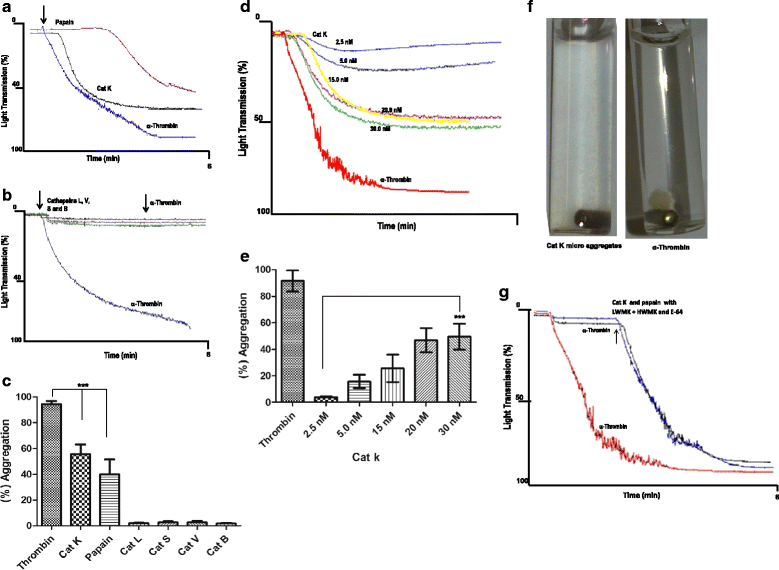
Results: We demonstrate that platelets are aggregated by cathepsin K in a dose-dependent manner, but not by other cysteine cathepsins. PARs-3 and -4 were confirmed as the cathepsin K target by immunodetection and specific antagonists using a fibroblast cell line derived from PARs deficient mice. Moreover, through co-culture experiments, we show that platelets activated by cathepsin K mediated the up-regulation of SHH, PTHrP, OPN, and TGFβ in epithelial-mesenchymal-like cells from patients with Luminal B breast cancer.
Conclusions: Cathepsin K induces platelet dysfunction and affects signaling in breast cancer cells.
Figures






Similar articles
-
Lysosomal protein turnover contributes to the acquisition of TGFβ-1 induced invasive properties of mammary cancer cells.Mol Cancer. 2015 Feb 15;14:39. doi: 10.1186/s12943-015-0313-5. Mol Cancer. 2015. PMID: 25744631 Free PMC article.
-
Neutrophil proteases can inactivate human PAR3 and abolish the co-receptor function of PAR3 on murine platelets.Thromb Haemost. 2001 Mar;85(3):533-8. Thromb Haemost. 2001. PMID: 11307827
-
Cathepsin G activates protease-activated receptor-4 in human platelets.J Biol Chem. 2000 Mar 10;275(10):6819-23. doi: 10.1074/jbc.275.10.6819. J Biol Chem. 2000. PMID: 10702240
-
The interaction of thrombin with blood platelets.Platelets. 2005 Nov;16(7):373-85. doi: 10.1080/09537100500123568. Platelets. 2005. PMID: 16236598 Review.
-
Obesity and Cathepsin K: A Complex Pathophysiological Relationship in Breast Cancer Metastases.Endocr Metab Immune Disord Drug Targets. 2020;20(8):1227-1231. doi: 10.2174/1871530320666200505115132. Endocr Metab Immune Disord Drug Targets. 2020. PMID: 32368981 Review.
Cited by
-
Identifying Methylation Pattern and Genes Associated with Breast Cancer Subtypes.Int J Mol Sci. 2019 Aug 31;20(17):4269. doi: 10.3390/ijms20174269. Int J Mol Sci. 2019. PMID: 31480430 Free PMC article.
-
Impacts of Cancer on Platelet Production, Activation and Education and Mechanisms of Cancer-Associated Thrombosis.Cancers (Basel). 2018 Nov 14;10(11):441. doi: 10.3390/cancers10110441. Cancers (Basel). 2018. PMID: 30441823 Free PMC article. Review.
-
Cysteine Cathepsins in Breast Cancer: Promising Targets for Fluorescence-Guided Surgery.Mol Imaging Biol. 2023 Feb;25(1):58-73. doi: 10.1007/s11307-022-01768-4. Epub 2022 Aug 24. Mol Imaging Biol. 2023. PMID: 36002710 Free PMC article. Review.
-
Interface between breast cancer cells and the tumor microenvironment using platelet-rich plasma to promote tumor angiogenesis - influence of platelets and fibrin bundles on the behavior of breast tumor cells.Oncotarget. 2017 Mar 7;8(10):16851-16874. doi: 10.18632/oncotarget.15170. Oncotarget. 2017. PMID: 28187434 Free PMC article.
-
Cathepsin K in Pathological Conditions and New Therapeutic and Diagnostic Perspectives.Int J Mol Sci. 2022 Nov 9;23(22):13762. doi: 10.3390/ijms232213762. Int J Mol Sci. 2022. PMID: 36430239 Free PMC article. Review.
References
-
- Mohamed MM, Sloane BF. Cysteine cathepsins: multifunctional enzymes in cancer. Nat Rev Cancer. 2006; doi:10.1038/nrc1949. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

