Intracranial EEG potentials estimated from MEG sources: A new approach to correlate MEG and iEEG data in epilepsy
- PMID: 26931511
- PMCID: PMC6867331
- DOI: 10.1002/hbm.23127
Intracranial EEG potentials estimated from MEG sources: A new approach to correlate MEG and iEEG data in epilepsy
Abstract
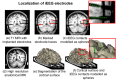
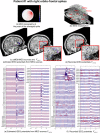
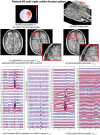
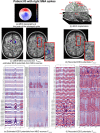
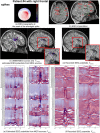
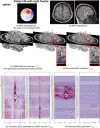
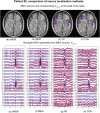
Detection of epileptic spikes in MagnetoEncephaloGraphy (MEG) requires synchronized neuronal activity over a minimum of 4cm2. We previously validated the Maximum Entropy on the Mean (MEM) as a source localization able to recover the spatial extent of the epileptic spike generators. The purpose of this study was to evaluate quantitatively, using intracranial EEG (iEEG), the spatial extent recovered from MEG sources by estimating iEEG potentials generated by these MEG sources. We evaluated five patients with focal epilepsy who had a pre-operative MEG acquisition and iEEG with MRI-compatible electrodes. Individual MEG epileptic spikes were localized along the cortical surface segmented from a pre-operative MRI, which was co-registered with the MRI obtained with iEEG electrodes in place for identification of iEEG contacts. An iEEG forward model estimated the influence of every dipolar source of the cortical surface on each iEEG contact. This iEEG forward model was applied to MEG sources to estimate iEEG potentials that would have been generated by these sources. MEG-estimated iEEG potentials were compared with measured iEEG potentials using four source localization methods: two variants of MEM and two standard methods equivalent to minimum norm and LORETA estimates. Our results demonstrated an excellent MEG/iEEG correspondence in the presumed focus for four out of five patients. In one patient, the deep generator identified in iEEG could not be localized in MEG. MEG-estimated iEEG potentials is a promising method to evaluate which MEG sources could be retrieved and validated with iEEG data, providing accurate results especially when applied to MEM localizations. Hum Brain Mapp 37:1661-1683, 2016. © 2016 Wiley Periodicals, Inc.
Keywords: epilepsy; epilepsy surgery; epileptic spikes; intracranial EEG; magnetic resonance imaging; magnetic source imaging; neurophysiology; presurgical investigation; source localization.
© 2016 Wiley Periodicals, Inc.
Figures
References
-
- Agirre‐Arrizubieta Z, Huiskamp GJ, Ferrier CH, van Huffelen AC, Leijten FS (2009): Interictal magnetoencephalography and the irritative zone in the electrocorticogram. Brain 132:3060–3071. - PubMed
-
- Amblard C, Lapalme E, Lina JM (2004): Biomagnetic source detection by maximum entropy and graphical models. IEEE Trans Biomed Eng 51:427–442. - PubMed
-
- Badier JM, Bartolomei F, Chauvel P, Bénar CG, Gavaret M (2015): Magnetic source imaging in posterior cortex epilepsies. Brain Topogr 28:162–171. - PubMed
-
- Bénar CG, Grova C, Kobayashi E, Bagshaw AP, Aghakhani Y, Dubeau F, Gotman J (2006): EEG‐fMRI of epileptic spikes: Concordance with EEG source localization and intracranial EEG. Neuroimage 30:1161–1170. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

