Transcriptional factor snail controls tumor neovascularization, growth and metastasis in mouse model of human ovarian carcinoma
- PMID: 26932374
- PMCID: PMC4884043
- DOI: 10.1186/s40169-014-0028-z
Transcriptional factor snail controls tumor neovascularization, growth and metastasis in mouse model of human ovarian carcinoma
Abstract
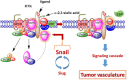
Background: Snail, a transcriptional factor and repressor of E-cadherin is well known for its role in cellular invasion. It can regulate epithelial to mesenchymal transition (EMT) during embryonic development and in epithelial cells. Snail also mediates tumor progression and metastases. Silencing of Snail and its associate member Slug in human A2780 ovarian epithelial carcinoma cell line was investigated to identify its role in tumor neovascularization.
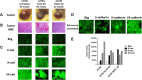
Methods: Live cell sialidase, WST-1 cell viability and immunohistochemistry assays were used to evaluate sialidase activity, cell survival and the expression levels of tumor E-cadherin, N-cadherin, VE-cadherin, and host endothelial CD31+(PECAM-1) cells in archived paraffin-embedded ovarian A2780, A2780 Snail shRNA GIPZ lentiviral knockdown (KD) and A2780 Slug shRNA GIPZ lentiviral KD tumors grown in RAGxCγ double mutant mice.
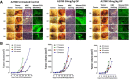
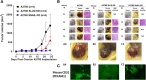
Results: Oseltamivir phosphate (OP), anti-Neu1 antibodies and MMP-9 specific inhibitor blocked Neu1 activity associated with epidermal growth factor (EGF) stimulated A2780 ovarian epithelial carcinoma cells. Silencing Snail in A2780 cells abrogated the Neu1 activity following EGF stimulation of the cells compared to A2780 and A2780 Slug KD cells. OP treatment of A2780 and cisplatin-resistant A2780cis cells reproducibly and dose-dependently abated the cell viability with a LD50 of 7 and 4 μm, respectively, after 48 h of incubation. Heterotopic xenografts of A2780 and A2780 Slug KD tumors developed robust and bloody tumor vascularization in RAG2xCγ double mutant mice. OP treatment at 50 mg/kg daily intraperitoneally did not significantly impede A2780 tumor growth rate but did cause a significant reduction of lung metastases compared with the untreated and OP 30mg/kg cohorts. Silencing Snail in A2780 tumor cells completely abrogated tumor vascularization, tumor growth and spread to the lungs in RAGxCγ double mutant mice. A2780 and A2780 Slug KD tumors expressed high levels of human N- and VE-cadherins, and host CD31+ endothelial cells, while A2780 Snail KD tumors expressed E-cadherin and reduced host CD31+ cells. OP 50mg/kg cohort tumors had reduced numbers of host CD31+ cells compared to a higher expression levels of CD31+ cells in tumors from the untreated control and OP 30mg/kg cohorts.
Conclusion: Snail transcriptional factor is an important intermediate player in human ovarian tumor neovascularization.
Keywords: Human ovarian cancer; Oseltamivir phosphate; Silencing transcriptional repressors Snail and Slug; Tumor neovascularization.
Figures

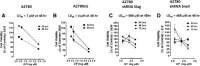
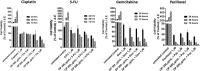


Similar articles
-
Therapeutic designed poly (lactic-co-glycolic acid) cylindrical oseltamivir phosphate-loaded implants impede tumor neovascularization, growth and metastasis in mouse model of human pancreatic carcinoma.Drug Des Devel Ther. 2015 Aug 10;9:4573-86. doi: 10.2147/DDDT.S90170. eCollection 2015. Drug Des Devel Ther. 2015. PMID: 26309402 Free PMC article.
-
Oseltamivir phosphate monotherapy ablates tumor neovascularization, growth, and metastasis in mouse model of human triple-negative breast adenocarcinoma.Breast Cancer (Dove Med Press). 2014 Dec 9;6:191-203. doi: 10.2147/BCTT.S74663. eCollection 2014. Breast Cancer (Dove Med Press). 2014. PMID: 25525387 Free PMC article.
-
Therapeutic targeting of Neu1 sialidase with oseltamivir phosphate (Tamiflu®) disables cancer cell survival in human pancreatic cancer with acquired chemoresistance.Onco Targets Ther. 2014 Jan 16;7:117-34. doi: 10.2147/OTT.S55344. eCollection 2014. Onco Targets Ther. 2014. PMID: 24470763 Free PMC article.
-
Inflammatory mediators drive metastasis and drug resistance in head and neck squamous cell carcinoma.Laryngoscope. 2015 Mar;125 Suppl 3:S1-11. doi: 10.1002/lary.24998. Epub 2015 Feb 3. Laryngoscope. 2015. PMID: 25646683
-
Changes in the expression of E-cadherin repressors, Snail, Slug, SIP1, and Twist, in the development and progression of ovarian carcinoma: the important role of Snail in ovarian tumorigenesis and progression.Med Mol Morphol. 2009 Jun;42(2):82-91. doi: 10.1007/s00795-008-0436-5. Epub 2009 Jun 18. Med Mol Morphol. 2009. PMID: 19536615
Cited by
-
Therapeutic designed poly (lactic-co-glycolic acid) cylindrical oseltamivir phosphate-loaded implants impede tumor neovascularization, growth and metastasis in mouse model of human pancreatic carcinoma.Drug Des Devel Ther. 2015 Aug 10;9:4573-86. doi: 10.2147/DDDT.S90170. eCollection 2015. Drug Des Devel Ther. 2015. PMID: 26309402 Free PMC article.
-
Agonist-Biased Signaling via Matrix Metalloproteinase-9 Promotes Extracellular Matrix Remodeling.Cells. 2018 Aug 26;7(9):117. doi: 10.3390/cells7090117. Cells. 2018. PMID: 30149671 Free PMC article. Review.
-
Oseltamivir phosphate monotherapy ablates tumor neovascularization, growth, and metastasis in mouse model of human triple-negative breast adenocarcinoma.Breast Cancer (Dove Med Press). 2014 Dec 9;6:191-203. doi: 10.2147/BCTT.S74663. eCollection 2014. Breast Cancer (Dove Med Press). 2014. PMID: 25525387 Free PMC article.
-
A Triple Combination of Metformin, Acetylsalicylic Acid, and Oseltamivir Phosphate Impacts Tumour Spheroid Viability and Upends Chemoresistance in Triple-Negative Breast Cancer.Drug Des Devel Ther. 2020 May 25;14:1995-2019. doi: 10.2147/DDDT.S242514. eCollection 2020. Drug Des Devel Ther. 2020. PMID: 32546966 Free PMC article.
-
Dynamic cell culture modulates colon cancer cell migration in a novel 3D cell culture system.Sci Rep. 2024 Aug 14;14(1):18851. doi: 10.1038/s41598-024-69261-2. Sci Rep. 2024. PMID: 39143115 Free PMC article.
References
-
- Jayson GC, Kohn EC, Kitchener HC, Ledermann JA: Ovarian cancer. The Lancet 2014, corrected proof (DOI: 10.1016/S0140–6736(13)62146–7). Jayson GC, Kohn EC, Kitchener HC, Ledermann JA: Ovarian cancer.The Lancet 2014, corrected proof (DOI: 10.1016/S0140-6736(13)62146-7). - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
