Regulation of immunity during visceral Leishmania infection
- PMID: 26932389
- PMCID: PMC4774109
- DOI: 10.1186/s13071-016-1412-x
Regulation of immunity during visceral Leishmania infection
Abstract
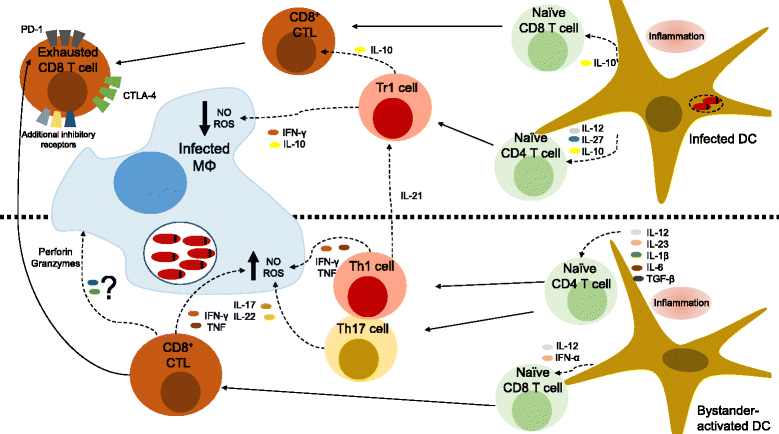
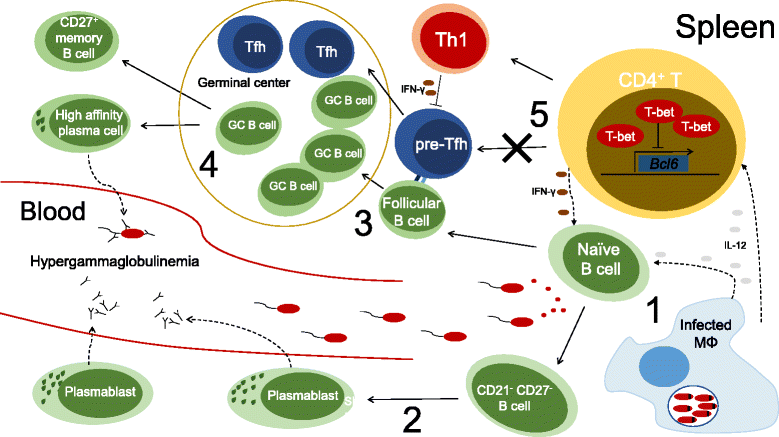
Unicellular eukaryotes of the genus Leishmania are collectively responsible for a heterogeneous group of diseases known as leishmaniasis. The visceral form of leishmaniasis, caused by L. donovani or L. infantum, is a devastating condition, claiming 20,000 to 40,000 lives annually, with particular incidence in some of the poorest regions of the world. Immunity to Leishmania depends on the development of protective type I immune responses capable of activating infected phagocytes to kill intracellular amastigotes. However, despite the induction of protective responses, disease progresses due to a multitude of factors that impede an optimal response. These include the action of suppressive cytokines, exhaustion of specific T cells, loss of lymphoid tissue architecture and a defective humoral response. We will review how these responses are orchestrated during the course of infection, including both early and chronic stages, focusing on the spleen and the liver, which are the main target organs of visceral Leishmania in the host. A comprehensive understanding of the immune events that occur during visceral Leishmania infection is crucial for the implementation of immunotherapeutic approaches that complement the current anti-Leishmania chemotherapy and the development of effective vaccines to prevent disease.
Figures
Comment in
-
Comment on "Regulation of immunity during visceral Leishmania infection" and further discussions about the role of antibodies in infections with Leishmania.Parasit Vectors. 2016 Jul 7;9(1):386. doi: 10.1186/s13071-016-1669-0. Parasit Vectors. 2016. PMID: 27387545 Free PMC article.
Similar articles
-
Immunity to visceral leishmaniasis: implications for immunotherapy.Future Microbiol. 2014;9(7):901-15. doi: 10.2217/fmb.14.43. Future Microbiol. 2014. PMID: 25156379 Review.
-
T-cell populations and cytokine expression are impaired in thymus and spleen of protein malnourished BALB/c mice infected with Leishmania infantum.PLoS One. 2014 Dec 23;9(12):e114584. doi: 10.1371/journal.pone.0114584. eCollection 2014. PLoS One. 2014. PMID: 25535967 Free PMC article.
-
IL-12 gene-deficient C57BL/6 mice are susceptible to Leishmania donovani but have diminished hepatic immunopathology.Eur J Immunol. 2000 Mar;30(3):834-9. doi: 10.1002/1521-4141(200003)30:3<834::AID-IMMU834>3.0.CO;2-9. Eur J Immunol. 2000. PMID: 10741399
-
Parasite burden in hamsters infected with two different strains of leishmania (Leishmania) infantum: "Leishman Donovan units" versus real-time PCR.PLoS One. 2012;7(10):e47907. doi: 10.1371/journal.pone.0047907. Epub 2012 Oct 24. PLoS One. 2012. PMID: 23112869 Free PMC article.
-
Mechanisms of resistance and susceptibility to experimental visceral leishmaniosis: BALB/c mouse versus Syrian hamster model.Vet Res. 2011 Feb 23;42(1):39. doi: 10.1186/1297-9716-42-39. Vet Res. 2011. PMID: 21345200 Free PMC article. Review.
Cited by
-
EB1-3 Chain of IL-35 Along With TGF-β Synergistically Regulate Anti-leishmanial Immunity.Front Immunol. 2019 Apr 12;10:616. doi: 10.3389/fimmu.2019.00616. eCollection 2019. Front Immunol. 2019. PMID: 31031744 Free PMC article.
-
Dependence of Leishmania parasite on host derived ATP: an overview of extracellular nucleotide metabolism in parasite.J Parasit Dis. 2019 Mar;43(1):1-13. doi: 10.1007/s12639-018-1061-4. Epub 2018 Dec 1. J Parasit Dis. 2019. PMID: 30956439 Free PMC article. Review.
-
An integrated bioinformatic analysis of microarray datasets to identify biomarkers and miRNA-based regulatory networks in leishmaniasis.Sci Rep. 2024 Jun 5;14(1):12981. doi: 10.1038/s41598-024-63462-5. Sci Rep. 2024. PMID: 38839916 Free PMC article.
-
Therapeutic effect of ursolic acid in experimental visceral leishmaniasis.Int J Parasitol Drugs Drug Resist. 2017 Apr;7(1):1-11. doi: 10.1016/j.ijpddr.2016.12.002. Epub 2016 Dec 8. Int J Parasitol Drugs Drug Resist. 2017. PMID: 27984757 Free PMC article.
-
Non-human primates and Leishmania immunity.Cytokine X. 2020 Oct 12;2(4):100038. doi: 10.1016/j.cytox.2020.100038. eCollection 2020 Dec. Cytokine X. 2020. PMID: 33604562 Free PMC article.
References
-
- Control of the leishmaniasis: Report of a meeting of the WHO Expert Committee on the Control of Leishmaniases [http://whqlibdoc.who.int/trs/WHO_TRS_949_eng.pdf]
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

